正在加载图片...
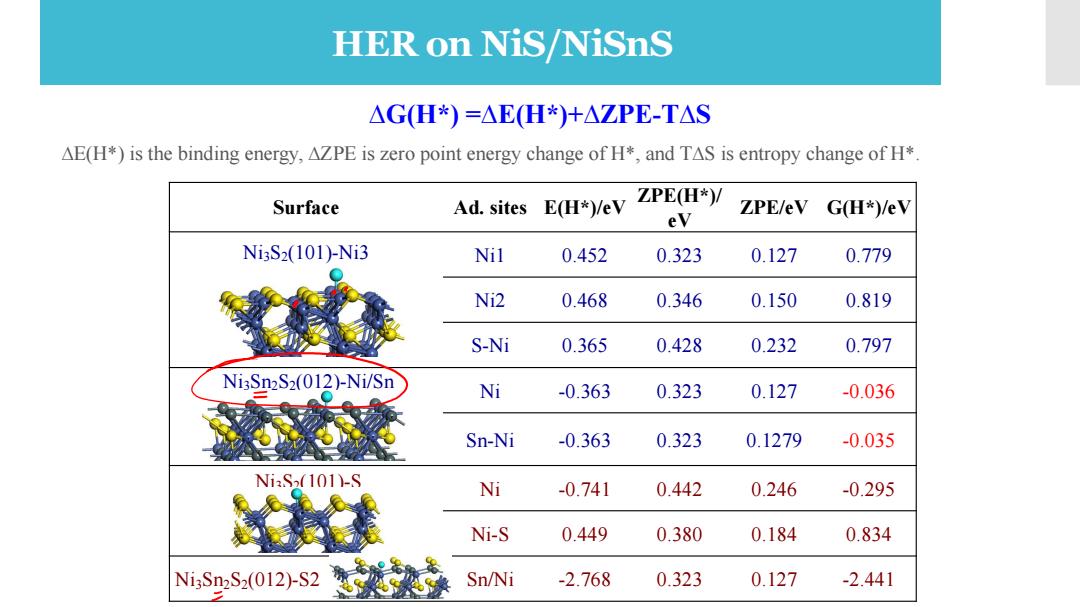
HER on NiS/NiSnS △GH*)=AEH*)+△ZPE-TAS AE(H*)is the binding energy,AZPE is zero point energy change of H*,and TAS is entropy change of H*. Surface Ad.sites E(H*)/eV ZPE(H*)/ ZPE/eV G(H*)/eV eV Ni3S2(101)-Ni3 Nil 0.452 0.323 0.127 0.779 Ni2 0.468 0.346 0.150 0.819 S-Ni 0.365 0.428 0.232 0.797 Ni3Sn2S2(012)-Ni/Sn Ni -0.363 0.323 0.127 -0.036 Sn-Ni -0.363 0.323 0.1279 -0.035 NiS(101)-S Ni -0.741 0.442 0.246 -0.295 Ni-S 0.449 0.380 0.184 0.834 Ni3Sn2S2(012)S2 Sn/Ni -2.768 0.323 0.127 -2.441Surface Ad. sites E(H*)/eV ZPE(H*)/ eV ZPE/eV G(H*)/eV Ni3S2(101)-Ni3 Ni1 0.452 0.323 0.127 0.779 Ni2 0.468 0.346 0.150 0.819 S-Ni 0.365 0.428 0.232 0.797 Ni3Sn2S2(012)-Ni/Sn Ni -0.363 0.323 0.127 -0.036 Sn-Ni -0.363 0.323 0.1279 -0.035 Ni3S2(101)-S Ni -0.741 0.442 0.246 -0.295 Ni-S 0.449 0.380 0.184 0.834 Ni3Sn2S2(012)-S2 Sn/Ni -2.768 0.323 0.127 -2.441 ∆G(H*) =∆E(H*)+∆ZPE-T∆S ∆E(H*) is the binding energy, ∆ZPE is zero point energy change of H*, and T∆S is entropy change of H*. The catalytic activity of Ni3S2 and Ni2Sn3S2 toward hydrogen evolution reaction HER on NiS/NiSnS