正在加载图片...
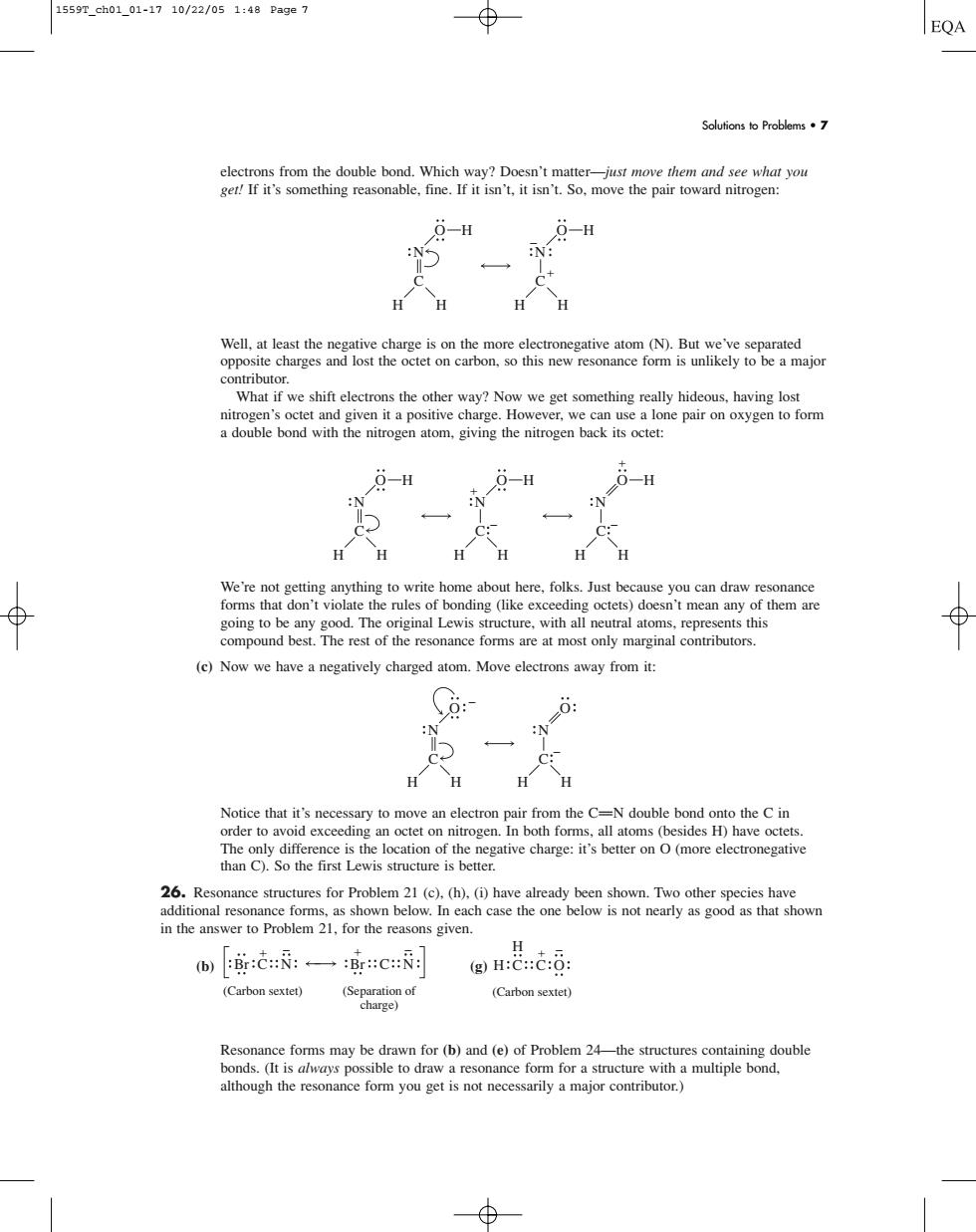
1559T_ch01_01-1710/22/051:48Page7 ⊕ EQA Solufions to Problems·7 s from the double bond.Which way?Doesn't ma ethem and see what you 9-H 9-H on oxygen ⊕ don't the rul 't mean any of them are in to b y of the resonance foms arat os rep (e)Now we have a negatively charged atom.Move electrons away from i 26.Resonance structures for Problem 21 (c).(h).(i)have already been shown.Two other species have 而::一减C H:C::C:0: (Carbon sextet) 6 Resonance forms may be drawn for (b)and (e)of Problem 24-the structures containing double electrons from the double bond. Which way? Doesn’t matter—just move them and see what you get! If it’s something reasonable, fine. If it isn’t, it isn’t. So, move the pair toward nitrogen: Well, at least the negative charge is on the more electronegative atom (N). But we’ve separated opposite charges and lost the octet on carbon, so this new resonance form is unlikely to be a major contributor. What if we shift electrons the other way? Now we get something really hideous, having lost nitrogen’s octet and given it a positive charge. However, we can use a lone pair on oxygen to form a double bond with the nitrogen atom, giving the nitrogen back its octet: We’re not getting anything to write home about here, folks. Just because you can draw resonance forms that don’t violate the rules of bonding (like exceeding octets) doesn’t mean any of them are going to be any good. The original Lewis structure, with all neutral atoms, represents this compound best. The rest of the resonance forms are at most only marginal contributors. (c) Now we have a negatively charged atom. Move electrons away from it: Notice that it’s necessary to move an electron pair from the CPN double bond onto the C in order to avoid exceeding an octet on nitrogen. In both forms, all atoms (besides H) have octets. The only difference is the location of the negative charge: it’s better on O (more electronegative than C). So the first Lewis structure is better. 26. Resonance structures for Problem 21 (c), (h), (i) have already been shown. Two other species have additional resonance forms, as shown below. In each case the one below is not nearly as good as that shown in the answer to Problem 21, for the reasons given. (b) (g) Resonance forms may be drawn for (b) and (e) of Problem 24—the structures containing double bonds. (It is always possible to draw a resonance form for a structure with a multiple bond, although the resonance form you get is not necessarily a major contributor.) H H C C O (Carbon sextet) Br C N N Br C (Separation of charge) (Carbon sextet) N H H O C N H H O C N H H O H C N H H O H C N H H H C O N H H O H C N H H O H C Solutions to Problems • 7 1559T_ch01_01-17 10/22/05 1:48 Page 7��������