正在加载图片...
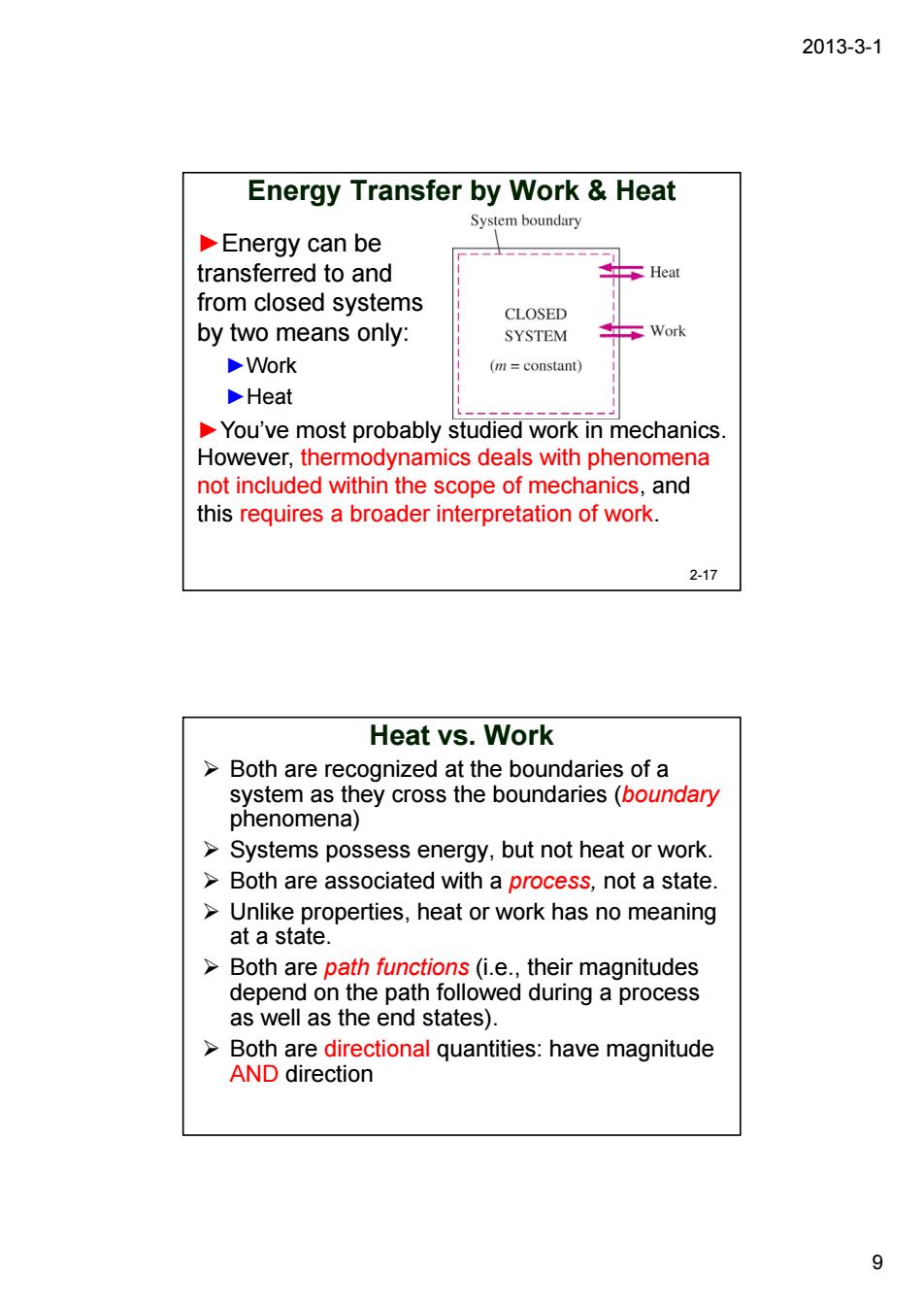
2013-3-1 Energy Transfer by Work Heat System boundary Energy can be transferred to and from closed systems CLOSED by two means only: SYSTEM Work Work (m=constant) Heat You've most probably studied work in mechanics. However,thermodynamics deals with phenomena not included within the scope of mechanics,and this requires a broader interpretation of work. 2-17 Heat vs.Work >Both are recognized at the boundaries of a 8g8tenaneycostnebounares6ouincen Systems possess energy,but not heat or work. >Both are associated with a process,not a state Unlike properties,heat or work has no meaning at a state. Both are path functions(i.e..their magnitudes depend on the path followed during a process as well as the end states). >Both are directional quantities:have magnitude AND direction 92013-3-1 9 Energy Transfer by Work & Heat ►Energy can be transferred to and from closed systems by two means only: ►Work ►Heat ►You’ve most probably studied work in mechanics. However, thermodynamics deals with phenomena not included within the scope of mechanics, and this requires a broader interpretation of work. 2-17 Heat vs. Work ¾ Both are recognized at the boundaries of a system as they cross the boundaries (boundary phenomena) ¾ Systems possess energy, but not heat or work. ¾ Both are associated with a process, not a state. ¾ Unlike properties, heat or work has no meaning at a state. ¾ Both are path functions (i.e., their magnitudes depend on the path followed during a process as well as the end states). ¾ Both are directional quantities: have magnitude AND direction