正在加载图片...
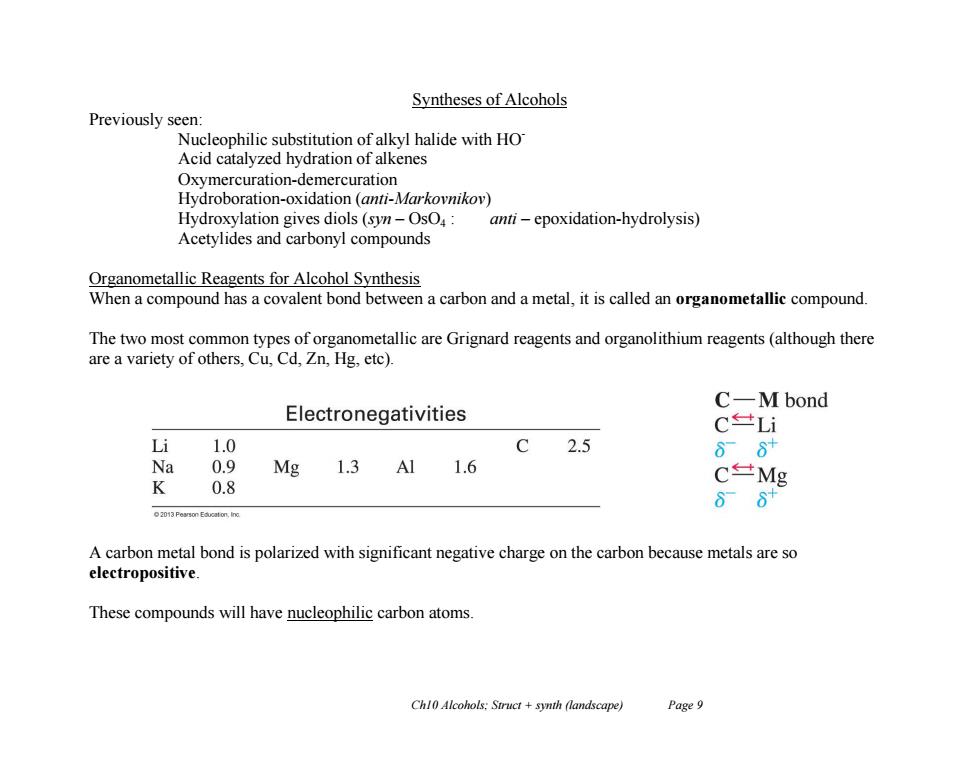
Syntheses of Alcohols Previously seen: Nucleophilic substitution of alkyl halide with HO Acid catalyzed hydration of alkenes Oxymercuration-demercuration Hydroboration-oxidation (anti-Markovnikov) Hydroxylation gives diols(syn-OsO:anti-epoxidation-hydrolysis) Acetylides and carbonyl compounds Organometallic Reagents for Alcohol Synthesis When a compound has a covalent bond between a carbon and a metal,it is called an organometallic compound. The two most common types of organometallic are Grignard reagents and organolithium reagents(although there are a variety of others,Cu,Cd,Zn,Hg,etc). C-M bond Electronegativities C世Li Li 1.0 C2.5 88 Na 0.9 Mg 1.3 A11.6 C±Mg K 0.8 86 2013 Pearson Education,Ino A carbon metal bond is polarized with significant negative charge on the carbon because metals are so electropositive These compounds will have nucleophilic carbon atoms. Chl0 Alcohols:Struct +synth (landscape) Page 9 Ch10 Alcohols; Struct + synth (landscape) Page 9 Syntheses of Alcohols Previously seen: Nucleophilic substitution of alkyl halide with HOAcid catalyzed hydration of alkenes Oxymercuration-demercuration Hydroboration-oxidation (anti-Markovnikov) Hydroxylation gives diols (syn – OsO4 : anti – epoxidation-hydrolysis) Acetylides and carbonyl compounds Organometallic Reagents for Alcohol Synthesis When a compound has a covalent bond between a carbon and a metal, it is called an organometallic compound. The two most common types of organometallic are Grignard reagents and organolithium reagents (although there are a variety of others, Cu, Cd, Zn, Hg, etc). A carbon metal bond is polarized with significant negative charge on the carbon because metals are so electropositive. These compounds will have nucleophilic carbon atoms