正在加载图片...
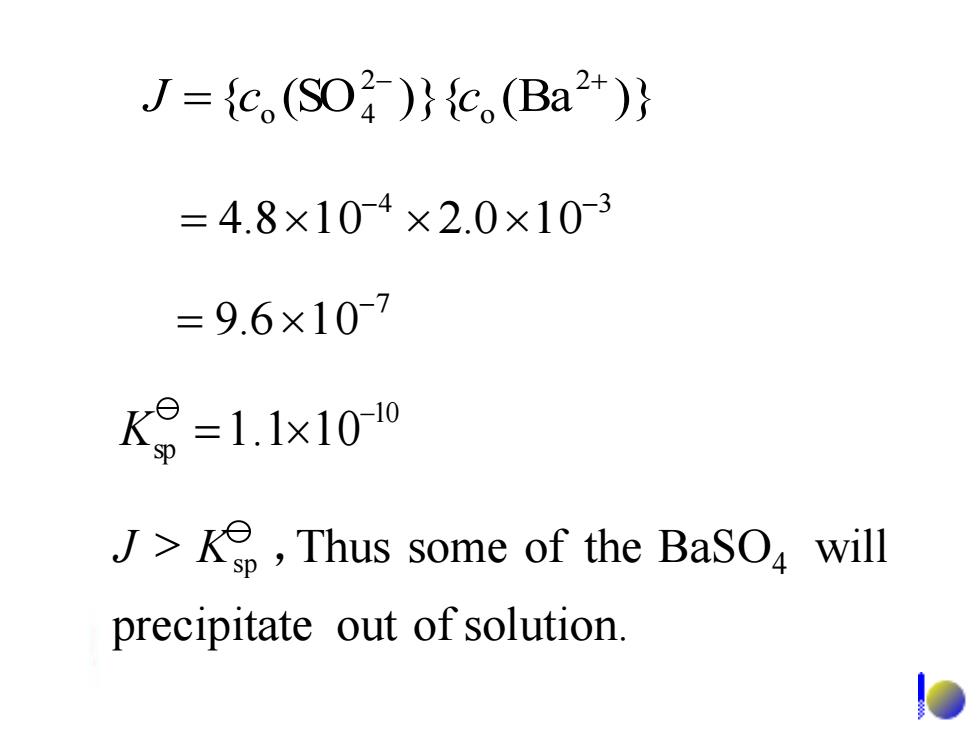
J={c(SO)}{c.(Ba2+)} =4.8×104×2.0×10-3 =9.6×10-7 K=1.1x100 J>Ke,Thus some of the BaSO will precipitate out of solution.7 9.6 10- = 4 3 4.8 1 0 2.0 1 0 - - = { (SO )}{ (Ba )} 2 o 2 o 4 - + J = c c 10 sp 1.1 10- K = precipitate out of solution. BaSO will 4 J > Ksp ,Thus some of the