正在加载图片...
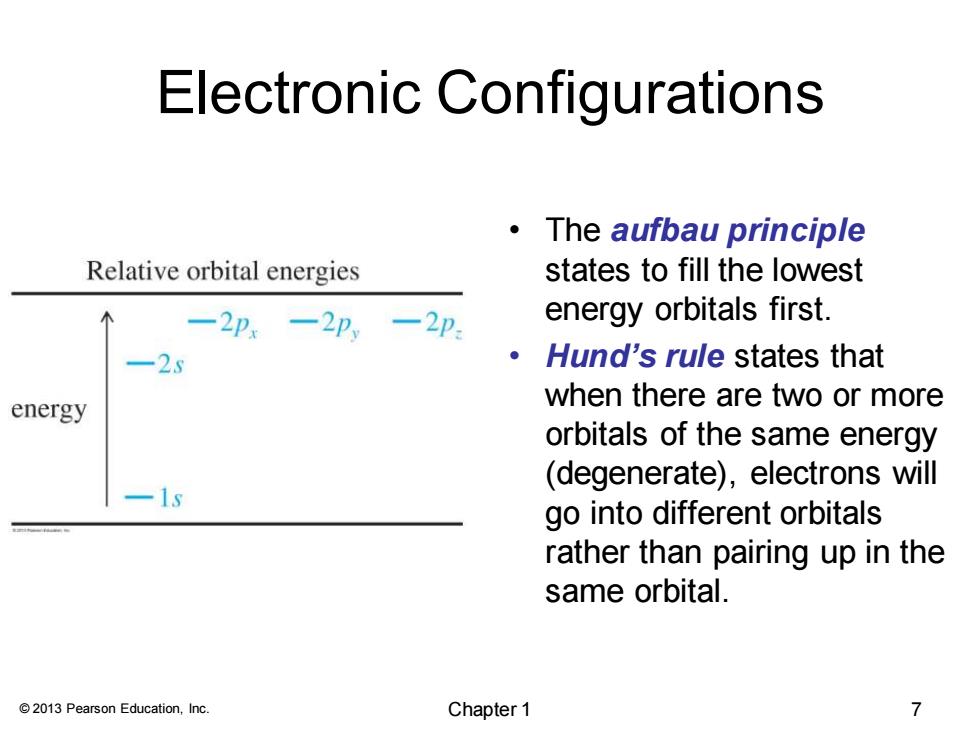
Electronic Configurations 。The aufbau principle Relative orbital energies states to fill the lowest 一2p,-2p一2p energy orbitals first -25 Hund's rule states that energy when there are two or more orbitals of the same energy (degenerate),electrons will -1s go into different orbitals rather than pairing up in the same orbital. 2013 Pearson Education,Inc. Chapter 1 7© 2013 Pearson Education, Inc. Electronic Configurations • The aufbau principle states to fill the lowest energy orbitals first. • Hund’s rule states that when there are two or more orbitals of the same energy (degenerate), electrons will go into different orbitals rather than pairing up in the same orbital. Chapter 1 7