正在加载图片...
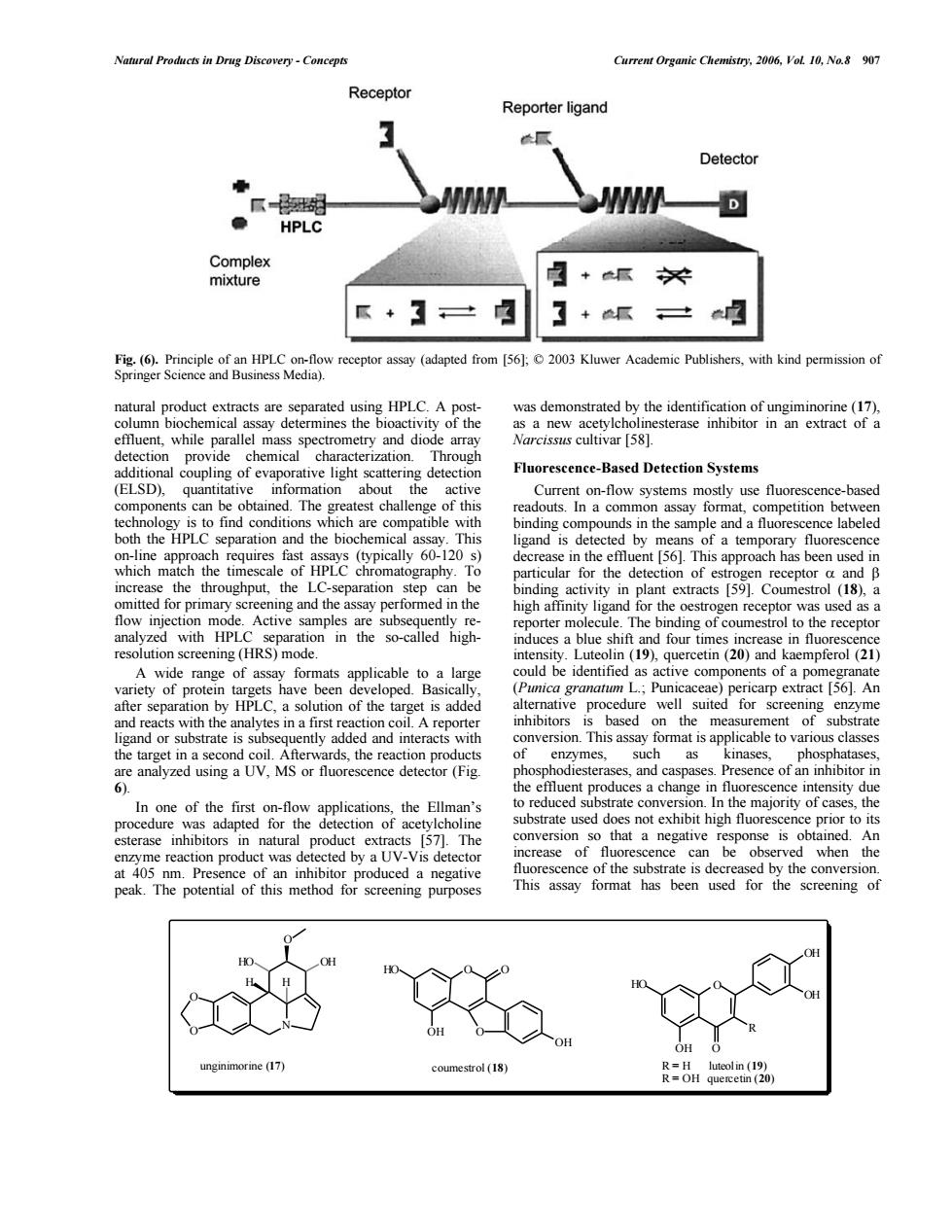
Natural Products in Drug Discovery-Concepts Current Organic Chemistry.2006.VoL 10,No.8 907 Receptor Reporter ligand Detector WI HPLC Complex mixture 瓜+3户 】+纸口 5gaaeom ceptor assay (adapted Kluwer Academic Publishers.with kind 。 n an extract of Fluorescence-Based Detection Systems qua act ligand is de ine the ef aoapp high for the oestrogen receptorwas as a resange with Rspmogon in the so-called high- of protein taeLC.a solution of the target iscally of the inhibitors and interac ap ds the such and caspases ence of the Ellman's te cor majority ht that a negative is of the ubstrate been use for 0 及:u4een120Natural Products in Drug Discovery - Concepts Current Organic Chemistry, 2006, Vol. 10, No.8 907 natural product extracts are separated using HPLC. A postcolumn biochemical assay determines the bioactivity of the effluent, while parallel mass spectrometry and diode array detection provide chemical characterization. Through additional coupling of evaporative light scattering detection (ELSD), quantitative information about the active components can be obtained. The greatest challenge of this technology is to find conditions which are compatible with both the HPLC separation and the biochemical assay. This on-line approach requires fast assays (typically 60-120 s) which match the timescale of HPLC chromatography. To increase the throughput, the LC-separation step can be omitted for primary screening and the assay performed in the flow injection mode. Active samples are subsequently reanalyzed with HPLC separation in the so-called highresolution screening (HRS) mode. A wide range of assay formats applicable to a large variety of protein targets have been developed. Basically, after separation by HPLC, a solution of the target is added and reacts with the analytes in a first reaction coil. A reporter ligand or substrate is subsequently added and interacts with the target in a second coil. Afterwards, the reaction products are analyzed using a UV, MS or fluorescence detector (Fig. 6). In one of the first on-flow applications, the Ellman’s procedure was adapted for the detection of acetylcholine esterase inhibitors in natural product extracts [57]. The enzyme reaction product was detected by a UV-Vis detector at 405 nm. Presence of an inhibitor produced a negative peak. The potential of this method for screening purposes was demonstrated by the identification of ungiminorine (17), as a new acetylcholinesterase inhibitor in an extract of a Narcissus cultivar [58]. Fluorescence-Based Detection Systems Current on-flow systems mostly use fluorescence-based readouts. In a common assay format, competition between binding compounds in the sample and a fluorescence labeled ligand is detected by means of a temporary fluorescence decrease in the effluent [56]. This approach has been used in particular for the detection of estrogen receptor a and b binding activity in plant extracts [59]. Coumestrol (18), a high affinity ligand for the oestrogen receptor was used as a reporter molecule. The binding of coumestrol to the receptor induces a blue shift and four times increase in fluorescence intensity. Luteolin (19), quercetin (20) and kaempferol (21) could be identified as active components of a pomegranate (Punica granatum L.; Punicaceae) pericarp extract [56]. An alternative procedure well suited for screening enzyme inhibitors is based on the measurement of substrate conversion. This assay format is applicable to various classes of enzymes, such as kinases, phosphatases, phosphodiesterases, and caspases. Presence of an inhibitor in the effluent produces a change in fluorescence intensity due to reduced substrate conversion. In the majority of cases, the substrate used does not exhibit high fluorescence prior to its conversion so that a negative response is obtained. An increase of fluorescence can be observed when the fluorescence of the substrate is decreased by the conversion. This assay format has been used for the screening of Fig. (6). Principle of an HPLC on-flow receptor assay (adapted from [56]; © 2003 Kluwer Academic Publishers, with kind permission of Springer Science and Business Media). O O N O H HO OH H unginimorine (17) O OH O OH HO O coumestrol (18) O O HO OH OH OH R R = H luteolin (19) R = OH quercetin (20)