正在加载图片...
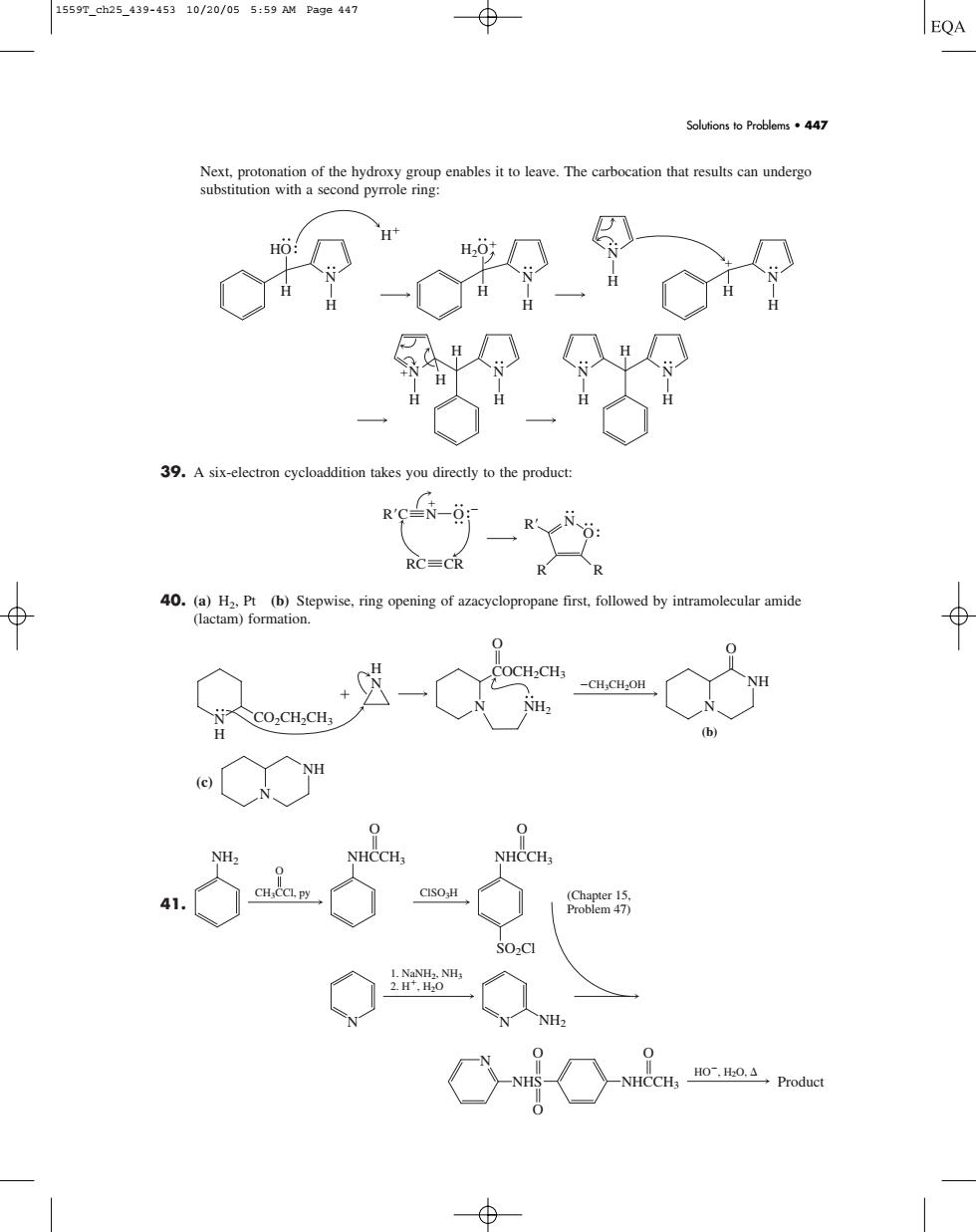
1559T_ch25_439-45310/20/055:59 AM Page447 EQA nables it to leave.The carbocation that results can underg 39.Asix-electron cycloaddition takes you directly to the product: R'C-N-0: 40.(a)H2.Pt (b)Step propane first followed by intramolecuar amide (lactam)formation S02C1 Next, protonation of the hydroxy group enables it to leave. The carbocation that results can undergo substitution with a second pyrrole ring: 39. A six-electron cycloaddition takes you directly to the product: 40. (a) H2, Pt (b) Stepwise, ring opening of azacyclopropane first, followed by intramolecular amide (lactam) formation. (c) 41. NHS HO, H2O, O O NHCCH3 O N Product NH2 NHCCH3 CH3CCl, py ClSO3H 1. NaNH2, NH3 2. H, H2O O O NHCCH3 SO2Cl O (Chapter 15, Problem 47) N N NH2 N NH COCH2CH3 CH3CH2OH CO2CH2CH3 O O NH2 N H H N N N NH (b) N O O R C R R R N RC CR N H N H H H H N N H H H N H H HO N H N H H H2O N H H Solutions to Problems • 447 1559T_ch25_439-453 10/20/05 5:59 AM Page 447