正在加载图片...
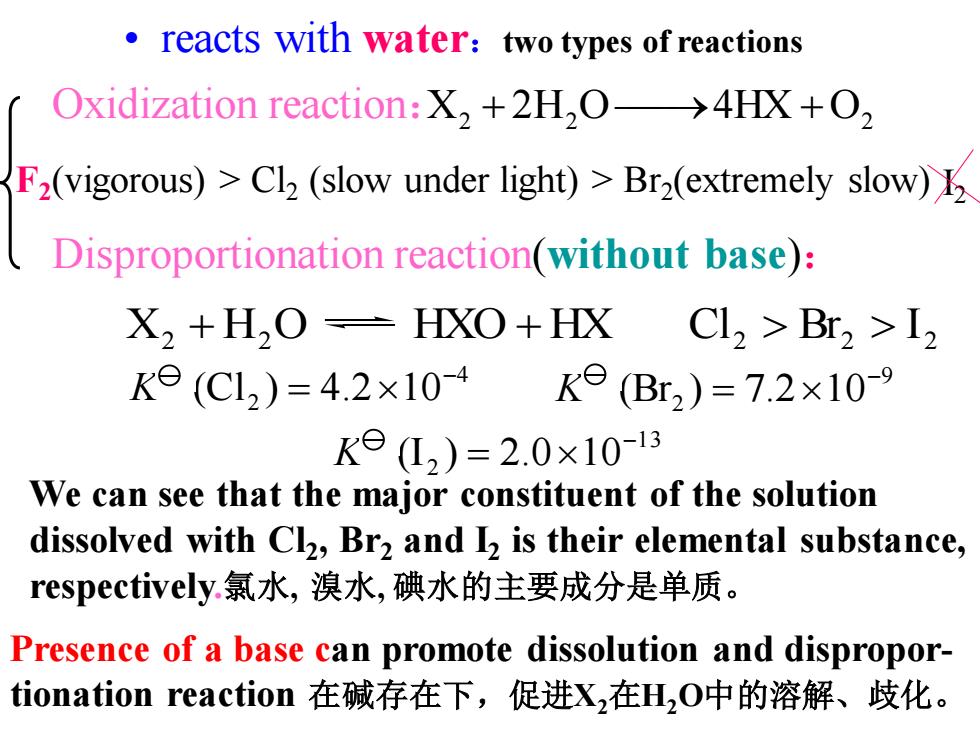
reacts with water:two types of reactions Oxidization reaction:X,+2H,O->4HX+O, F2(vigorous)>Cl(slow under light)>Br2(extremely slow) Disproportionation reaction(without base): X2 +H2O=HXO+HX Cl2 >Br2 >I Ke(C12)=4.2×10-4Ke(B2)=7.2×10-9 KeL2)=2.0×10-13 We can see that the major constituent of the solution dissolved with Cl,Br2 and I is their elemental substance, respectively.氯水,溴水,碘水的主要成分是单质。 Presence of a base can promote dissolution and dispropor- tionation reaction在碱存在下,促进X,在H2O中的溶解、歧化。X2 + 2H2 O ⎯→4HX + O2 Disproportionation reaction(without base): 2 2 2 Cl Br I We can see that the major constituent of the solution dissolved with Cl2 , Br2 and I2 is their elemental substance, respectively.氯水, 溴水, 碘水的主要成分是单质。 Presence of a base can promote dissolution and disproportionation reaction 在碱存在下,促进X2在H2O中的溶解、歧化。 • reacts with water:two types of reactions Oxidization reaction: 4 (Cl2 ) 4.2 10- K = 9 (Br2 ) 7.2 10- K = 13 (I2 ) 2.0 10- K = X2 + H2 O HXO + HX F2 (vigorous) > Cl2 (slow under light) > Br2 (extremely slow) I2