正在加载图片...
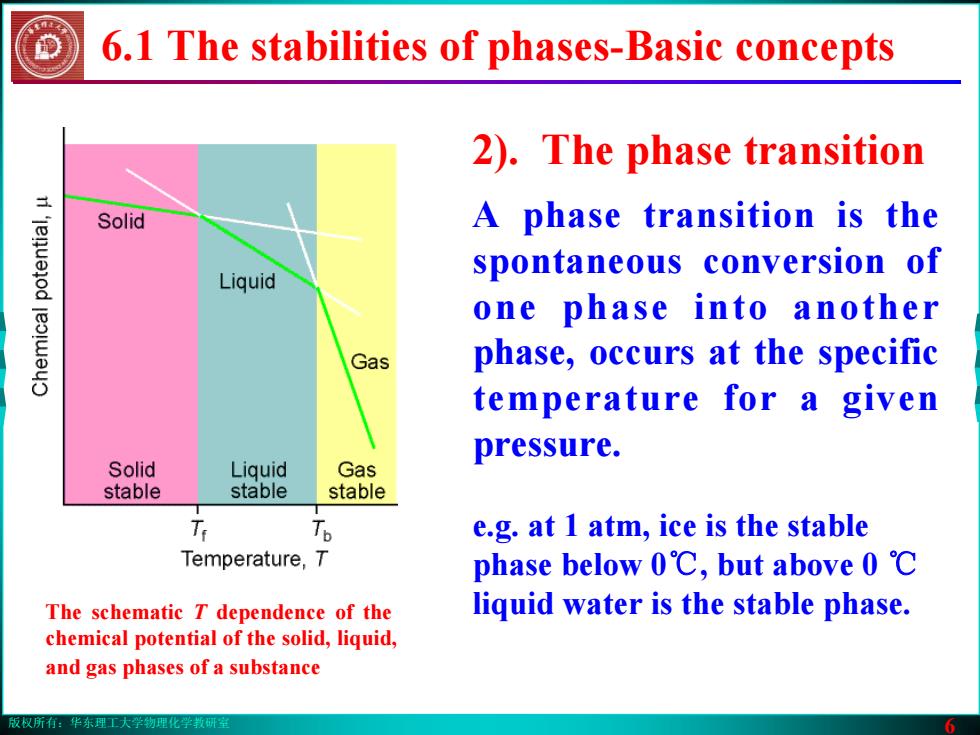
版权所有:华东理工大学物理化学教研室 6 6.1 The stabilities of phases-Basic concepts 2). The phase transition A phase transition is the spontaneous conversion of one phase into another phase, occurs at the specific temperature for a given pressure. e.g. at 1 atm, ice is the stable phase below 0℃, but above 0 ℃ The schematic T dependence of the liquid water is the stable phase. chemical potential of the solid, liquid, and gas phases of a substance版权所有:华东理工大学物理化学教研室 6 6.1 The stabilities of phases-Basic concepts 2). The phase transition A phase transition is the spontaneous conversion of one phase into another phase, occurs at the specific temperature for a given pressure. e.g. at 1 atm, ice is the stable phase below 0℃, but above 0 ℃ The schematic T dependence of the liquid water is the stable phase. chemical potential of the solid, liquid, and gas phases of a substance