正在加载图片...
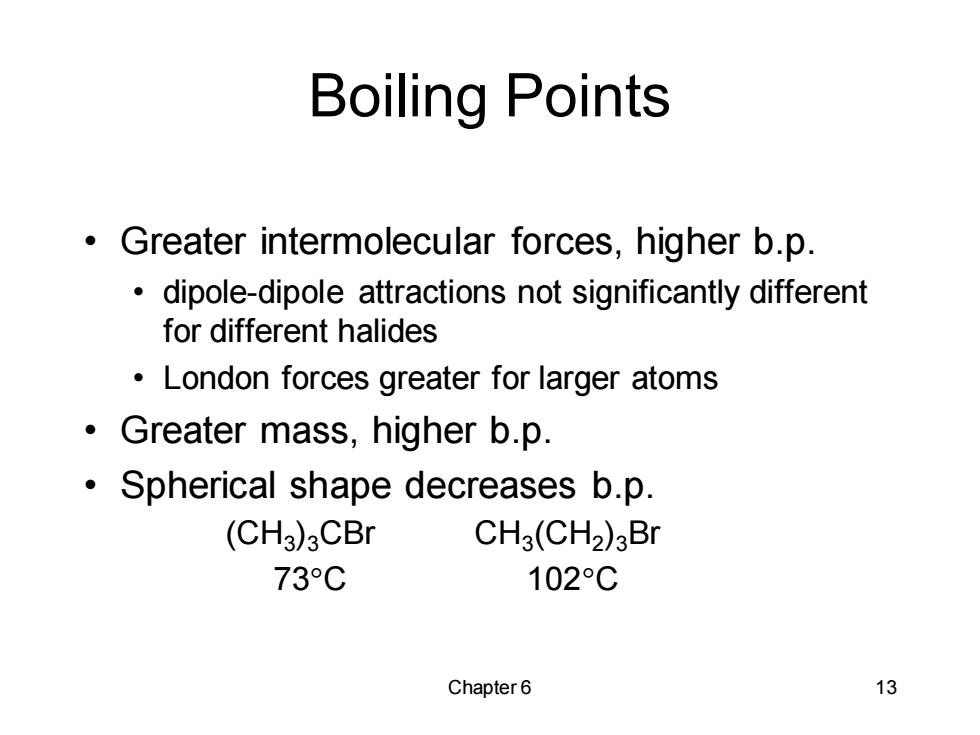
Boiling Points Greater intermolecular forces,higher b.p. dipole-dipole attractions not significantly different for different halides London forces greater for larger atoms Greater mass,higher b.p. Spherical shape decreases b.p. (CH3)3CBr CH3(CH2)3Br 73C 102C Chapter6 13Chapter 6 13 Boiling Points • Greater intermolecular forces, higher b.p. • dipole-dipole attractions not significantly different for different halides • London forces greater for larger atoms • Greater mass, higher b.p. • Spherical shape decreases b.p. (CH3 )3CBr CH3 (CH2 )3Br 73C 102C