正在加载图片...
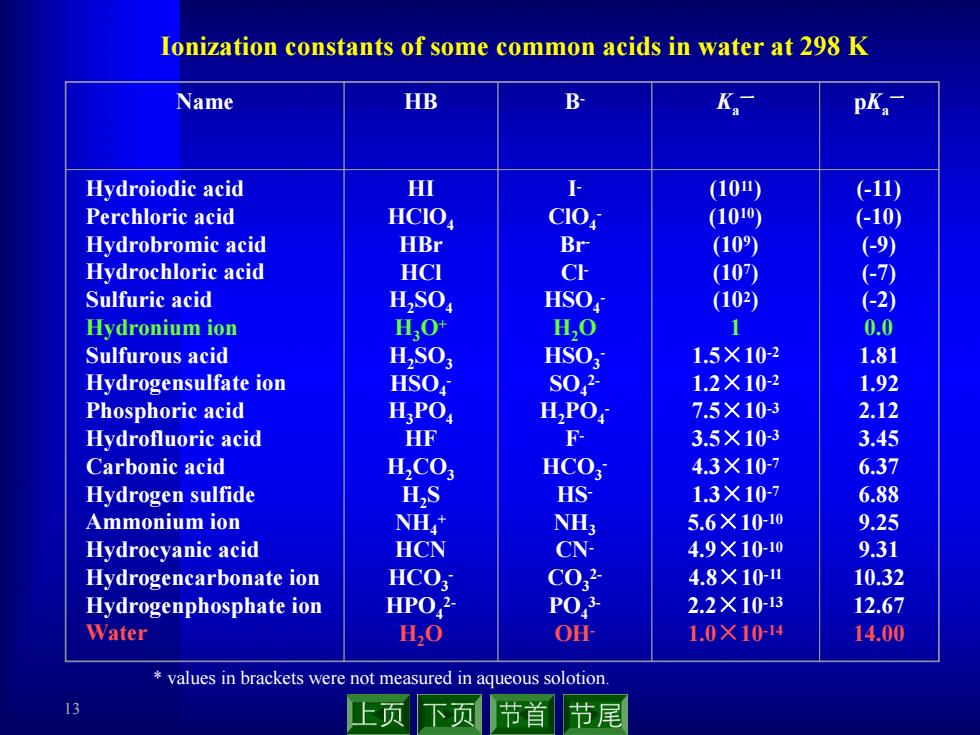
Ionization constants of some common acids in water at 298 K Name HB B K pK. Hydroiodic acid HI (10) (-11) Perchloric acid HCIO CIO (1010) (-10) Hydrobromic acid HBr Br (10) (9) Hydrochloric acid HCI (107) ←7) Sulfuric acid HSO HSO (102 (-2) Hydronium ion HO H,O 1 0.0 Sulfurous acid H,SO: HSO 1.5×10-2 1.81 Hydrogensulfate ion HSO S02 1.2×10-2 1.92 Phosphoric acid H.PO HPO 7.5×10-3 2.12 Hydrofluoric acid HF 3.5×103 3.45 Carbonic acid H,CO HCO 4.3×10-7 6.37 Hydrogen sulfide H,S HS 1.3X10-7 6.88 Ammonium ion NH NH; 5.6×10-10 9.25 Hydrocyanic acid HCN CN- 4.9×10-10 9.31 Hydrogencarbonate ion HCO; C032 4.8×10 10.32 Hydrogenphosphate ion HPO,2 P03 2.2×1013 12.67 Water H,O OH 1.0×1014 14.00 values in brackets were not measured in aqueous solotion 下页 节首 节尾 13 Ionization constants of some common acids in water at 298 K * values in brackets were not measured in aqueous solotion. Name HB B- Ka一 pKa一 Hydroiodic acid Perchloric acid Hydrobromic acid Hydrochloric acid Sulfuric acid Hydronium ion Sulfurous acid Hydrogensulfate ion Phosphoric acid Hydrofluoric acid Carbonic acid Hydrogen sulfide Ammonium ion Hydrocyanic acid Hydrogencarbonate ion Hydrogenphosphate ion Water HI HClO4 HBr HCl H2SO4 H3O+ H2SO3 HSO4 - H3PO4 HF H2CO3 H2S NH4 + HCN HCO3 - HPO4 2- H2O I - ClO4 - Br - Cl - HSO4 - H2O HSO3 - SO4 2- H2PO4 - F- HCO3 - HS- NH3 CN- CO3 2- PO4 3- OH- (1011) (1010) (109) (107) (102) 1 1.5×10-2 1.2×10-2 7.5×10-3 3.5×10-3 4.3×10-7 1.3×10-7 5.6×10-10 4.9×10-10 4.8×10-11 2.2×10-13 1.0×10-14 (-11) (-10) (-9) (-7) (-2) 0.0 1.81 1.92 2.12 3.45 6.37 6.88 9.25 9.31 10.32 12.67 14.00