正在加载图片...
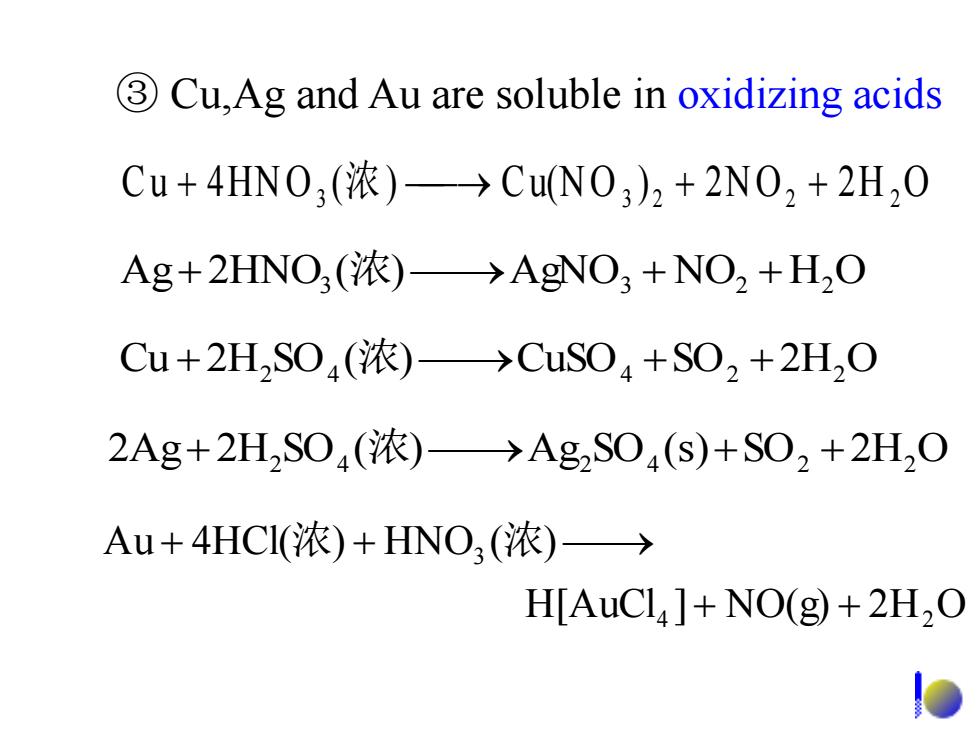
3 Cu,Ag and Au are soluble in oxidizing acids Cu+4HN0,(浓)→CuN0,)2+2N02+2H20 Ag+2HNO,(浓)→AgNO3+NO2+HO Cu+2HS04(浓)→CuS04+S02+2H20 2Ag+2H2S04(浓)→Ag2S04(s)+S02+2H2O Au+4HCI(浓)+HNO3(浓)→ H[AuCl]+NO(g)+2H2O ③ Cu,Ag and Au are soluble in oxidizing acids C u 4HN O3 (浓) Cu(N O 3 ) 2 2N O2 2 H 2 O H[AuCl ] NO(g) 2H O Au 4HCl( ) HNO ( ) 4 2 3 浓 浓 2Ag 2H2 SO4 (浓) Ag2 SO4 (s)SO2 2H2 O Cu 2H2 SO4 (浓) CuSO4 SO2 2H2 O Ag 2HNO3 (浓) AgNO3 NO2 H2 O