正在加载图片...
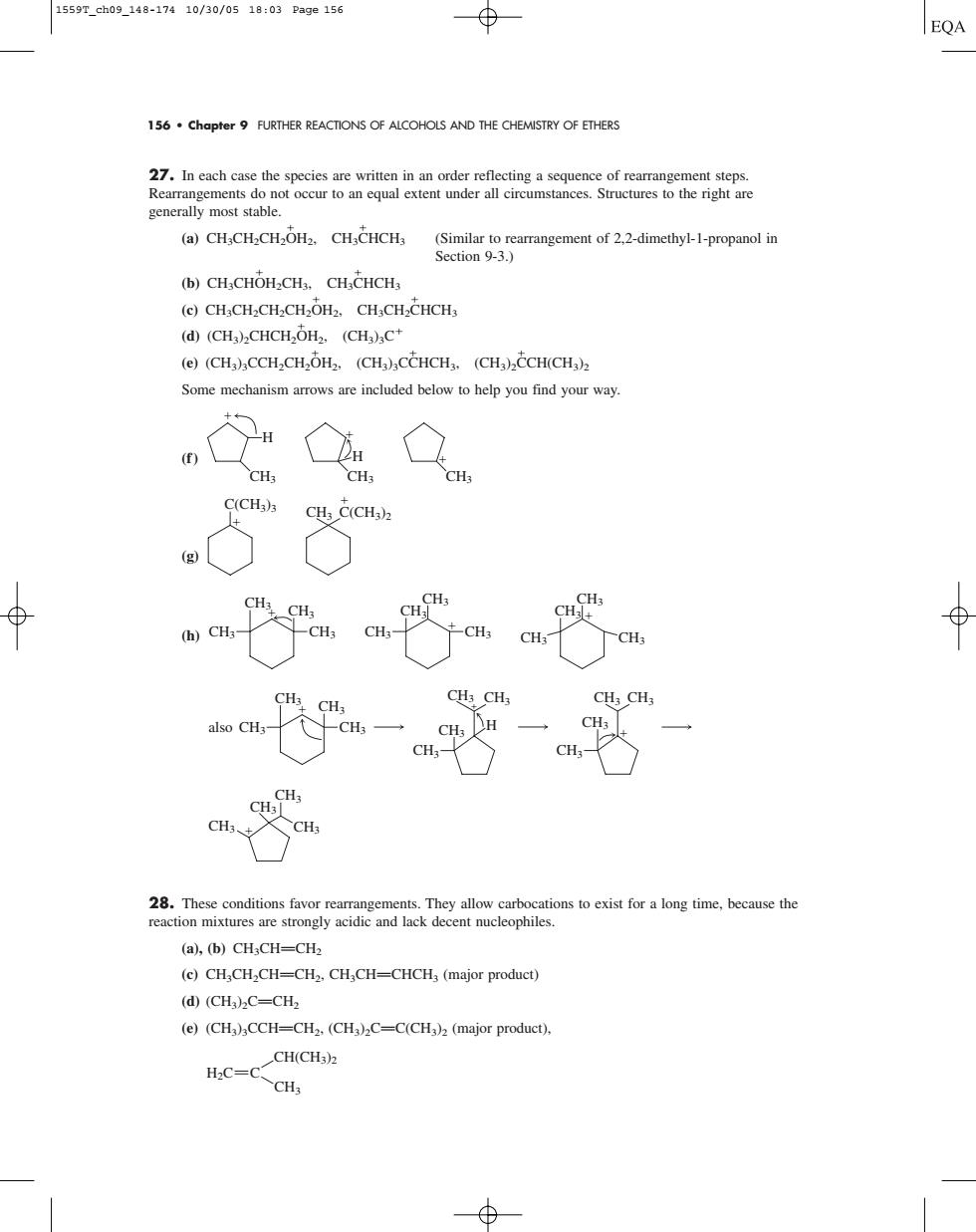
1559r_eh09_148-17410/30/0518:03Page156 EQA 156.chapter9 FURTHER REACTIONS OF ALCOHOLS AND THE CHEMISTRY OF ETHERS 27.In each case the species are written in an order reflecting a sequence of rearrangement steps. 一st心udrtructres to the h (a)CH,CHCHOH3.CH,CHCHs (Similar to rearrangement of 2.2-dimethyl-1-propanol in Section 9-3. (b)CH CHOH-CH,CH CHCHs (e)CH;CH2CH2CH2OH2.CH,CH2CHCH3 (d)(CH)2CHCH,OH2.(CH)C* (e)(CH)CCHCH,OH,.(CHCCHCH.(CHCCH(CH) Some mechanism arrows are included below to help you find your way CH; H C(CH3)3 CH C(CHa)2 ⊕ (h)CH3 -CH CH CH CHs CH:CH CH:- CH; CHs- CH ons to exist for a long time,because the (a),(b)CH;CH-CH2 (c)CH,CH.CH-CH2.CH,CH-CHCH3(major product) (d)(CH:C=CH2 (e)(CH3)aCCH-CH2.(CH3)2C-C(CH3)2(major product). CH(CH H2C=C CH3156 • Chapter 9 FURTHER REACTIONS OF ALCOHOLS AND THE CHEMISTRY OF ETHERS 27. In each case the species are written in an order reflecting a sequence of rearrangement steps. Rearrangements do not occur to an equal extent under all circumstances. Structures to the right are generally most stable. (a) CH3CH2CH2 OH2, CH3 CHCH3 (Similar to rearrangement of 2,2-dimethyl-1-propanol in Section 9-3.) (b) CH3CH OH2CH3, CH3 CHCH3 (c) CH3CH2CH2CH2 OH2, CH3CH2 CHCH3 (d) (CH3)2CHCH2 OH2, (CH3)3C (e) (CH3)3CCH2CH2 OH2, (CH3)3C CHCH3, (CH3)2 CCH(CH3)2 Some mechanism arrows are included below to help you find your way. (f ) (g) (h) 28. These conditions favor rearrangements. They allow carbocations to exist for a long time, because the reaction mixtures are strongly acidic and lack decent nucleophiles. (a), (b) CH3CHPCH2 (c) CH3CH2CHPCH2, CH3CHPCHCH3 (major product) (d) (CH3)2CPCH2 (e) (CH3)3CCHPCH2, (CH3)2CPC(CH3)2 (major product), CH3 H2C C CH(CH3)2 CH3 CH3 CH3 CH3 also CH3 CH3 CH3 CH3 CH3 H CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 C(CH3)3 CH3 C(CH3)2 CH3 H CH3 H CH3 1559T_ch09_148-174 10/30/05 18:03 Page 156�����������������������