正在加载图片...
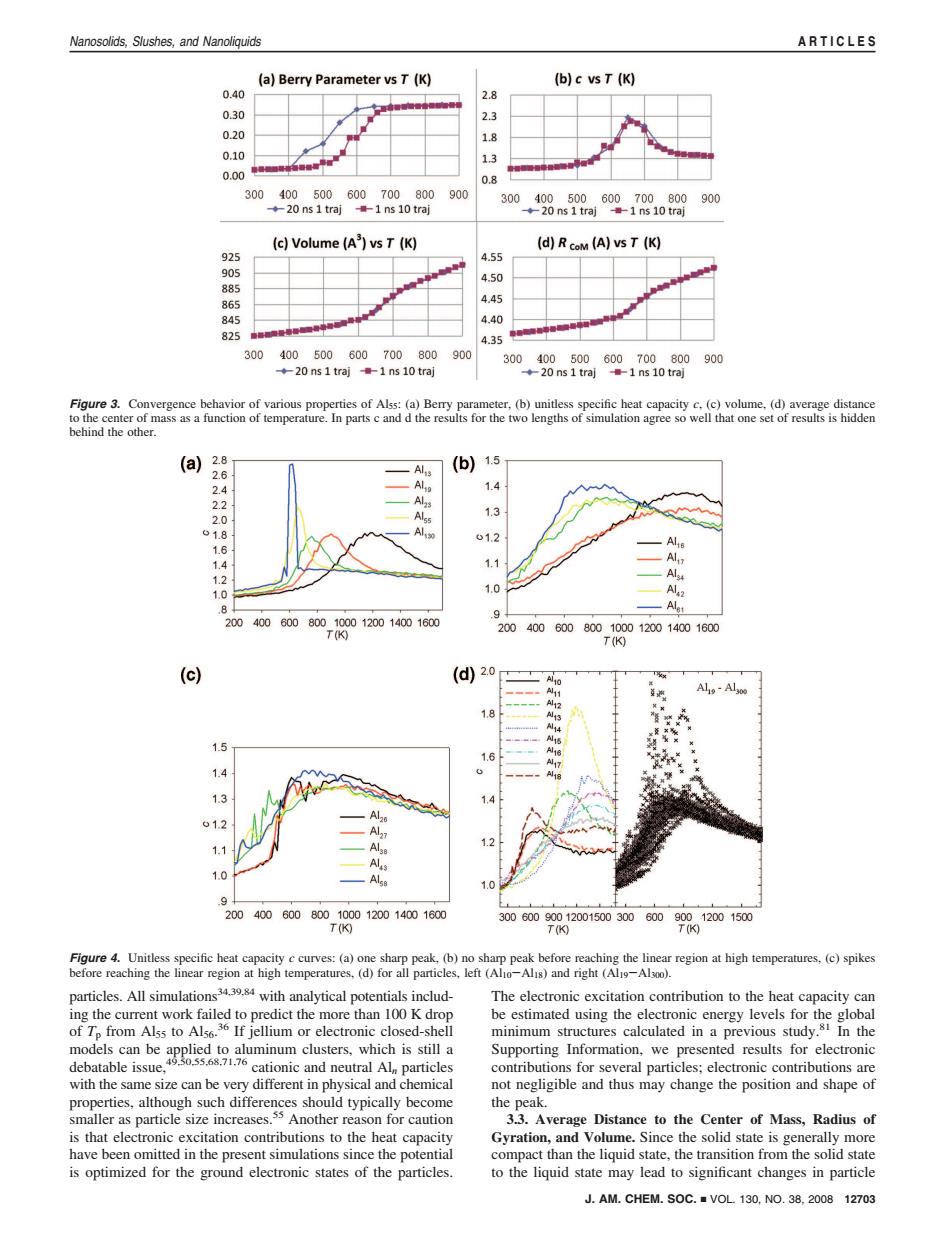
Nanosolids,Slushes,and Nanoliquids ARTICLES (a)Berry Parameter vs T(K) (b)c vs T (K) 0.40 2.8 0.30 0.20 1.8 0.10 13 0.00 p 300400500600700800 900 300400500600700800900 ◆20ns1traj1ns10traj 20 ns 1 traj-1 ns 10 traj (c)Volume(A)vs T(K) (d)RcoM (A)vs T (K) 925 4.55 905 4.50 885 865 4.45 845 4.40 825 4.35 300400500600700800 900 300400500600700800 900 ◆20ns1traj1ns10tra 20 ns 1 traj -1 ns 10 traj Figure 3.Convergence behavior of various properties of Alss:(a)Berry parameter,(b)unitless specific heat capacity c.(c)volume,(d)average distance to the center of mass as a function of temperature.In parts c and d the results for the two lengths of simulation agree so well that one set of results is hidden behind the other. (a) 2.8 (b) 1.5 2.6 2.4 1.4 2.2 2.0 Alss 1.3 01.8 A 01.2 1.6 1.4 1.1 1.2 1.0 1.0 8 2004006008001000120014001600 Alet T(K) 2004006008001000120014001600 T(K) (c) (d2.o A10 Al-Al30 12 1.8 15 16 A17 14 1 73 7.4 01.2 13 1.1 1.0 1.0 9 2004006008001000120014001600 3006009001200150030060090012001500 T(K) T(0 T(K) Figure 4.Unitless specific heat capacity c curves:(a)one sharp peak.(b)no sharp peak before reaching the linear region at high temperatures,(c)spikes before reaching the linear region at high temperatures,(d)for all particles,left (Alo-Alis)and right(Al19-Al3oo). particles.All simulations with analytical potentials includ- The electronic excitation contribution to the heat capacity can ing the current work failed to predict the more than 100 K drop be estimated using the electronic energy levels for the global of Tp from Alss to Als6.36 If jellium or electronic closed-shell minimum structures calculated in a previous study.81 In the ltaee n eue Supporting Information,we presented results for electronic debatable issue, contributions for several particles;electronic contributions are with the same size can be very different in physical and chemical not negligible and thus may change the position and shape of properties,although such differences should typically become the peak. smaller as particle size increases.ss Another reason for caution 3.3.Average Distance to the Center of Mass,Radius of is that electronic excitation contributions to the heat capacity Gyration,and Volume.Since the solid state is generally more have been omitted in the present simulations since the potential compact than the liquid state,the transition from the solid state is optimized for the ground electronic states of the particles. to the liquid state may lead to significant changes in particle J.AM.CHEM.SOC.VOL 130,NO.38,2008 12703particles. All simulations34,39,84 with analytical potentials including the current work failed to predict the more than 100 K drop of Tp from Al55 to Al56. 36 If jellium or electronic closed-shell models can be applied to aluminum clusters, which is still a debatable issue,49,50,55,68,71,76 cationic and neutral Aln particles with the same size can be very different in physical and chemical properties, although such differences should typically become smaller as particle size increases.55 Another reason for caution is that electronic excitation contributions to the heat capacity have been omitted in the present simulations since the potential is optimized for the ground electronic states of the particles. The electronic excitation contribution to the heat capacity can be estimated using the electronic energy levels for the global minimum structures calculated in a previous study.81 In the Supporting Information, we presented results for electronic contributions for several particles; electronic contributions are not negligible and thus may change the position and shape of the peak. 3.3. Average Distance to the Center of Mass, Radius of Gyration, and Volume. Since the solid state is generally more compact than the liquid state, the transition from the solid state to the liquid state may lead to significant changes in particle Figure 3. Convergence behavior of various properties of Al55: (a) Berry parameter, (b) unitless specific heat capacity c, (c) volume, (d) average distance to the center of mass as a function of temperature. In parts c and d the results for the two lengths of simulation agree so well that one set of results is hidden behind the other. Figure 4. Unitless specific heat capacity c curves: (a) one sharp peak, (b) no sharp peak before reaching the linear region at high temperatures, (c) spikes before reaching the linear region at high temperatures, (d) for all particles, left (Al10-Al18) and right (Al19-Al300). J. AM. CHEM. SOC. 9 VOL. 130, NO. 38, 2008 12703 Nanosolids, Slushes, and Nanoliquids ARTICLES