正在加载图片...
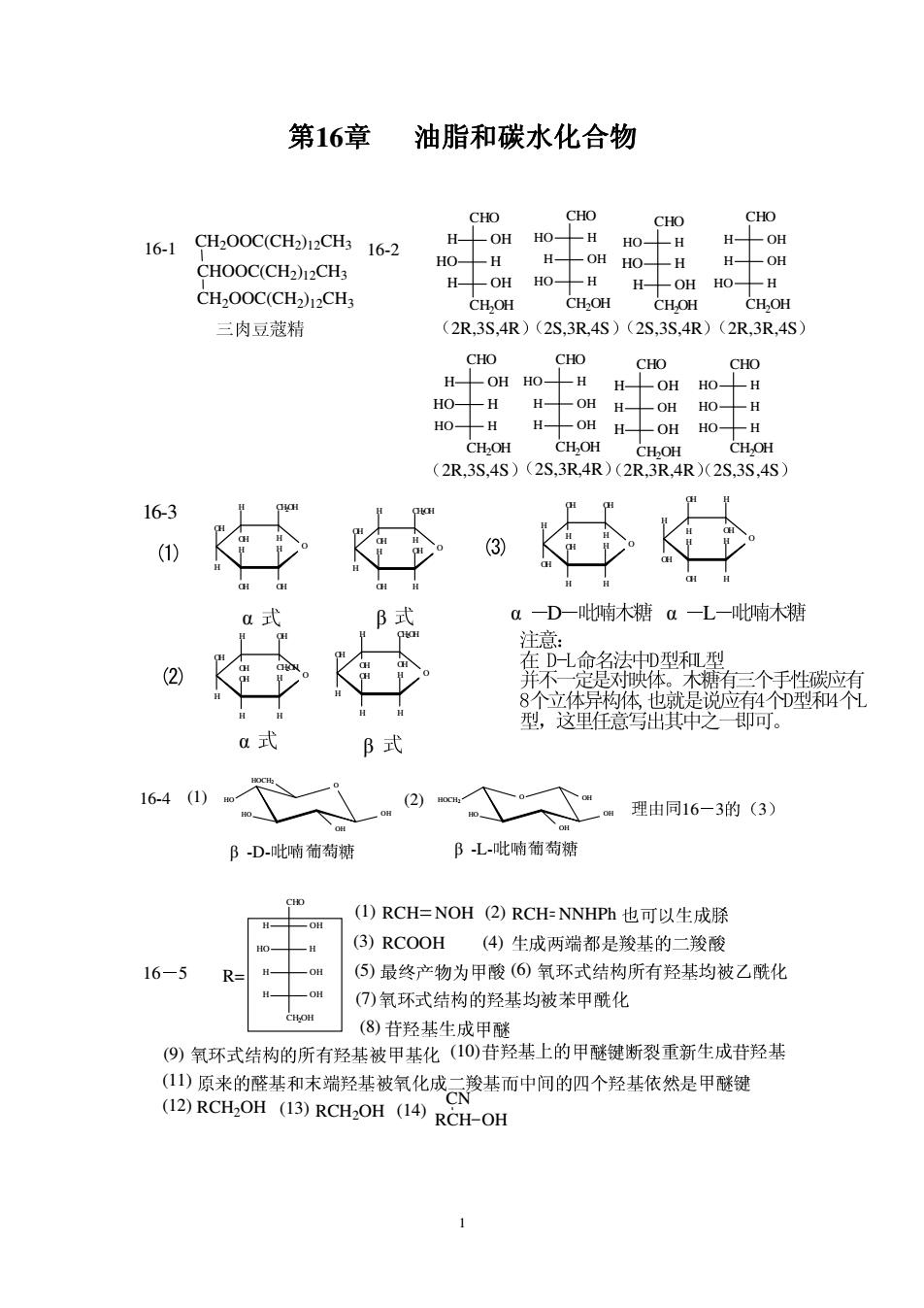
第16章油脂和碳水化合物 CHO CHO 16-1qH,00CCHh12CH16-2 H OH HO- CHOOC(CH2)12CH3 HO--H H-OH HO-H CH2OOC(CH)CH CH.OH CHOH 三肉豆蔻精 (2R,3S,4R)(2S.3R4S)(2S.3S.4R)(2R,3R,4S CHO CHO CHO CHO H-LOH HO- -H H HOH H- -OH HO-H H-OH CH.OH CH.OH CH.OH (2R.3S.4S)(2S,3R4R)(2R3R.4R)(2S.3S,4S) 16-3 a一D一吡喃木糖a一L一吡喃木糖 (2 并不 8个立体 型,这里任意写出其中之即可。 B式 16-4(1) (②)。人”m理由同16-3的(3) D吡啼葡萄糖 阝L-吡喃葡萄糖 (I)RCH=NOH(2)RCH-NNHPh也可以生成脎 (3)RCOOH(4)生成两端都是羧基的二羧酸 16-5 (⑤)最终产物为甲酸(⑥氧环式结构所有羟基均被乙酰化 (⑦)氧环式结构的羟基均被苯甲酰化 (8)苷羟基生成甲 (9)氧环式结构的所有羟基被甲基化(10)苷羟基上的甲醚键断裂重新生成苷羟基 1少原来的醛基和末端羟茱被氧化成云发基而中间的四个羟基依然是甲醚键 (12)RCH2OH (13)RCH2OH (14)RCH-OH 1 16
OH O H H OH OH H H CH2OH OH H OH O H OH OH H H H CH2OH OH H 16-3 OH O H H H H OH H CH2OH OH OH OH O H H H H OH H OH OH CH2OH H O OH H H H OH OH OH H H H O OH H OH H H OH H H OH D L
!
"#$%&'( )
)
*+,-./0
123 HO OH HOCH2 HO OH O O HO OH HOCH OH 2 OH -D-✂✁☎✄✝✆✟✞ -L-✂✁✟✄✠✆✟✞ 16-4 (1) (2) ✡☞☛✟✌16✍ 3✎✑✏ 3 ✒ 16✓5 CH2OH H OH H OH HO H H OH CHO R= (1) (2) (3) (5) (6) (4) RCH NOH RCH NNHPh ✔✖✕✘✗✟✙✝✚✖✛ RCOOH ✙✝✚✢✜✢✣✥✤✥✦★✧✖✩✫✪✟✬★✧✢✭ ✮✰✯★✱✢✲✴✳✫✵ ✭ ✶✥✷✥✸✰✹★✺✖✻✢✼✖✽✢✩✢✾✢✿❁❀✟❂✢❃ (7) ✶✥✷✥✸✰✹★✺❁✪❄✽✢✩✢✾✢✿✥❅ ✵ ❂✢❃ (8) ❆✽✢✩❇✙✠✚ ✵❉❈ (9) ✶✢✷✥✸✰✹★✺❁✪❊✻✢✼✖✽✢✩✢✿ ✵✩✥❃ (10)❆✽✢✩✢❋●✪ ✵❉❈✖❍❇■✠❏✥❑✢▲✙✠✚❆✽✢✩ (11) ▼★◆ ✪❊❖✖✩✥P✢◗✢✣✖✽✖✩✥✿✖✶✥❃✢✚✥✬✖✧✖✩✥❘●❙★❚✥✪✥❯❄❱✖✽✢✩✰❲✖❳✰✦ ✵❉❈✖❍ (12) RCH2OH (13) RCH2OH (14) RCH OH CN CH2OOC(CH2 )12CH3 CHOOC(CH2 )12CH3 CH2OOC(CH2 )12CH3 ❨❬❩❪❭✖❫✖❴ CH2OH H OH HO H H OH CHO CH2OH H OH HO H HO H CHO CH2OH HO H H OH H OH CHO CH2OH HO H H OH HO H CHO CH2OH H OH H OH H OH CHO CH2OH HO H HO H HO H CHO CH2OH HO H HO H H OH CHO CH2OH H OH H OH HO H CHO ❵ 2R,3S,4R ❛ ❵ 2S,3R,4S ❛ ❵ 2S,3S,4R ❛ ❵ 2R,3R,4S ❛ ❵ 2R,3R,4R ❛ ❵ 2S,3S,4S ❛ ❵ 2R,3S,4S ❛ ❵ 2S,3R,4R ❛ 16-1 16-2������������������