正在加载图片...
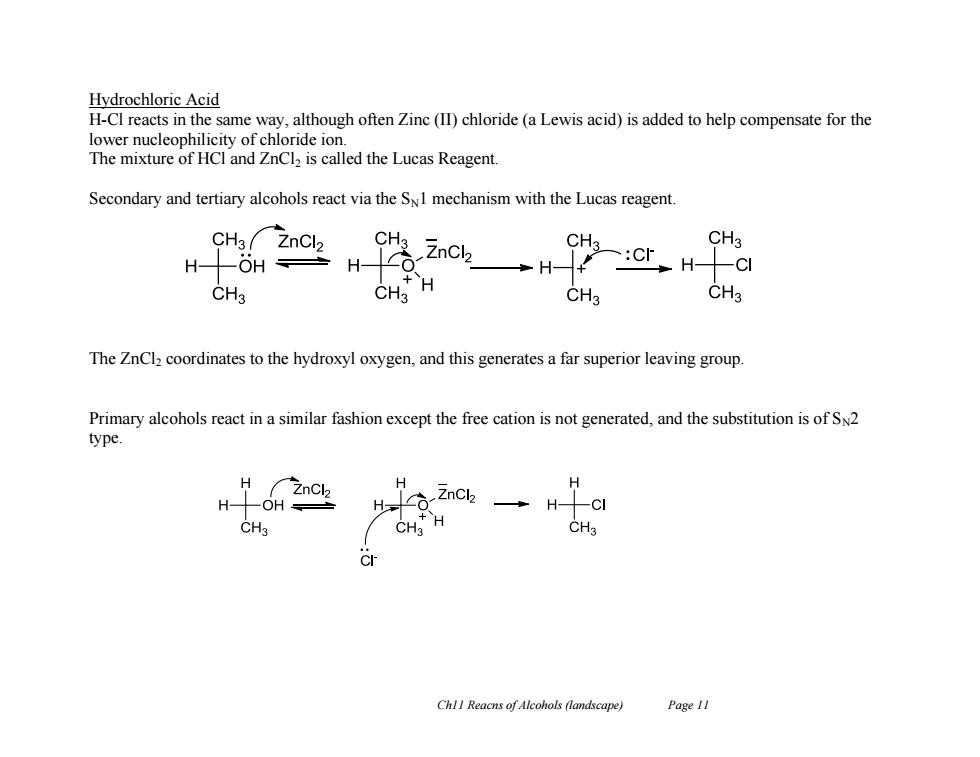
Hydrochloric Acid H-Cl reacts in the same way,although often Zinc (II)chloride(a Lewis acid)is added to help compensate for the lower nucleophilicity of chloride ion. The mixture of HCl and ZnCl2 is called the Lucas Reagent. Secondary and tertiary alcohols react via the S1 mechanism with the Lucas reagent. CH3 ZnCl2 CH ZnCl2 CH3 CH3 :CF H -OH÷H H CH3 CH3 H CH3 CH3 The ZnCl2 coordinates to the hydroxyl oxygen,and this generates a far superior leaving group. Primary alcohols react in a similar fashion except the free cation is not generated,and the substitution is of Sn2 type. H H -OH a2nch→Hcl CH3 CHa ChlI Reacns of Alcohols (landscape) Page 11Ch11 Reacns of Alcohols (landscape) Page 11 Hydrochloric Acid H-Cl reacts in the same way, although often Zinc (II) chloride (a Lewis acid) is added to help compensate for the lower nucleophilicity of chloride ion. The mixture of HCl and ZnCl2 is called the Lucas Reagent. Secondary and tertiary alcohols react via the SN1 mechanism with the Lucas reagent. The ZnCl2 coordinates to the hydroxyl oxygen, and this generates a far superior leaving group. Primary alcohols react in a similar fashion except the free cation is not generated, and the substitution is of SN2 type