正在加载图片...
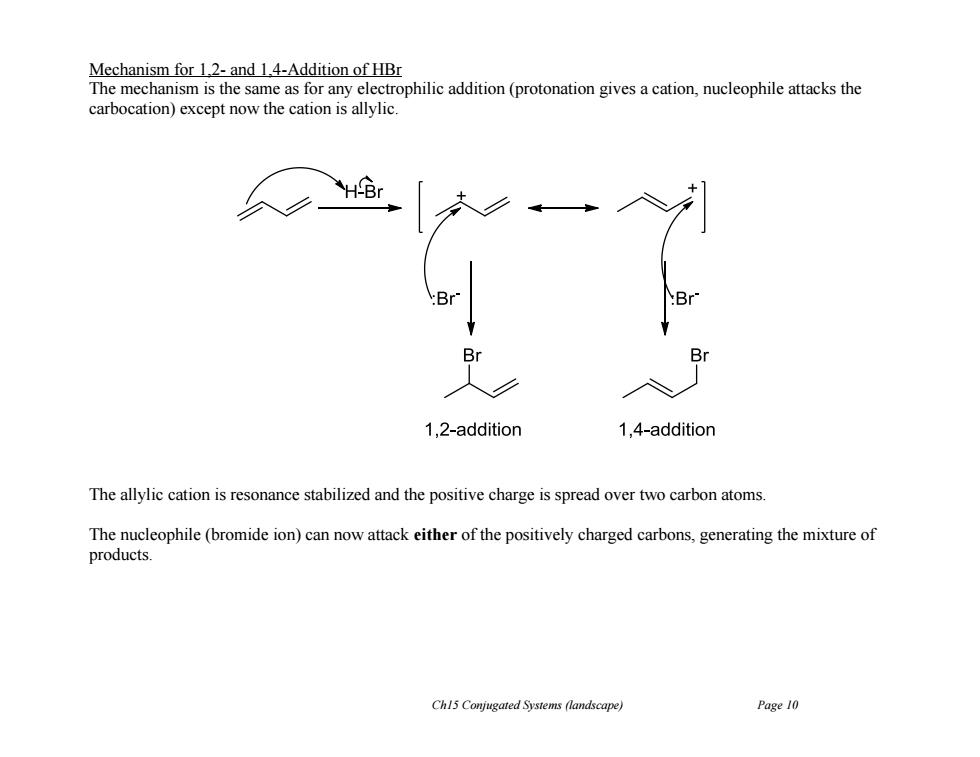
Mechanism for 1.2-and 1.4-Addition of HBr The mechanism is the same as for any electrophilic addition(protonation gives a cation,nucleophile attacks the carbocation)except now the cation is allylic. 1,2-addition 1,4-addition The allylic cation is resonance stabilized and the positive charge is spread over two carbon atoms. The nucleophile(bromide ion)can now attack either of the positively charged carbons,generating the mixture of products. Ch15 Conjugated Systems (landscape) Page 10Ch15 Conjugated Systems (landscape) Page 10 Mechanism for 1,2- and 1,4-Addition of HBr The mechanism is the same as for any electrophilic addition (protonation gives a cation, nucleophile attacks the carbocation) except now the cation is allylic. The allylic cation is resonance stabilized and the positive charge is spread over two carbon atoms. The nucleophile (bromide ion) can now attack either of the positively charged carbons, generating the mixture of products