正在加载图片...
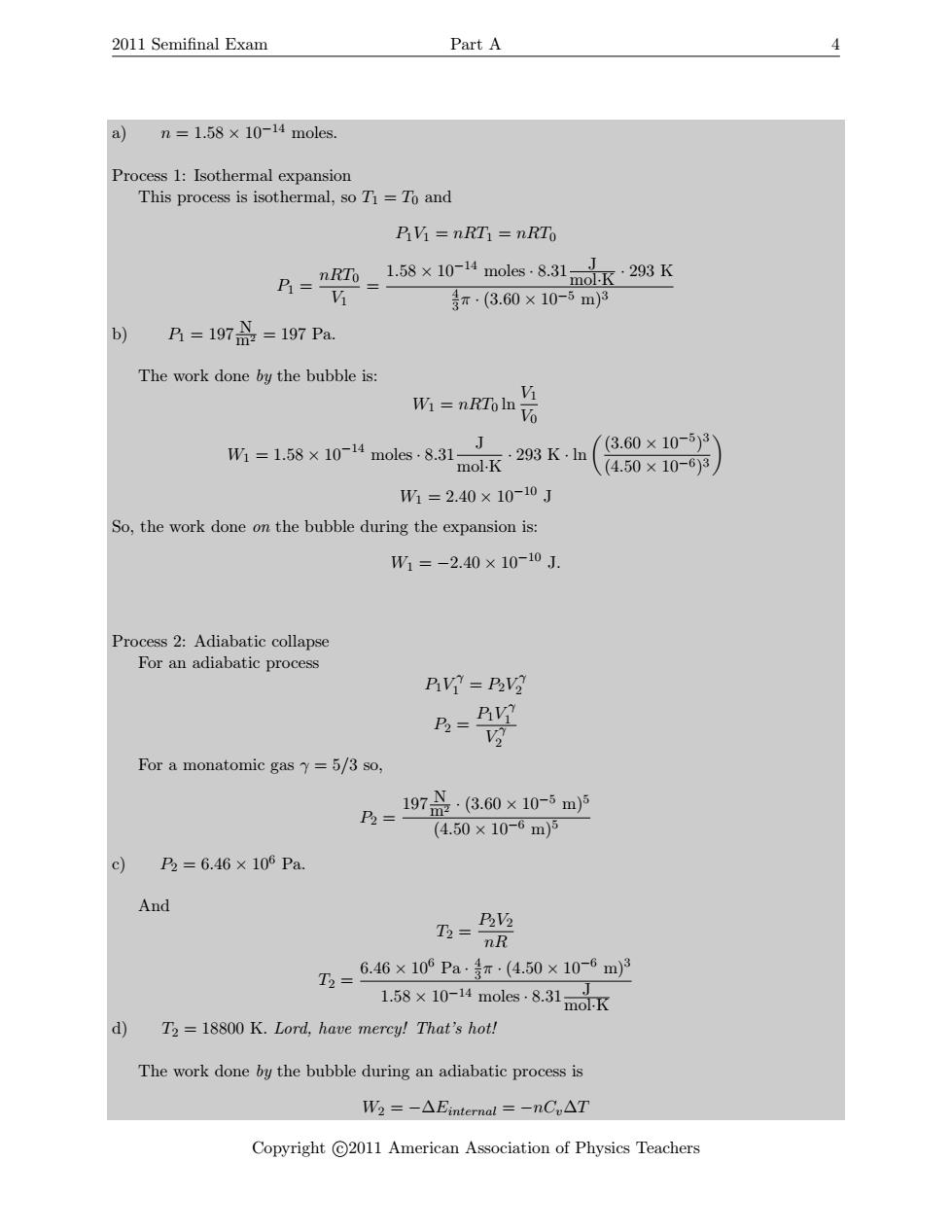
2011 Semifinal Exam Part A 4 a n=1.58×10-14 noles. Process 1:Isothermal expansion This process is isothermal,so Ti=To and 乃M=nRT1=nRTo B=nm=1.58×10-14 moles831nJ moR·293K 巧 等r·(3.60×10-5m3 b) A=1970=197Pa. The work done by the bubble is: W1 nRTo ln M 6 W=1.58×10-14 moles8.31-J .293K,1n (3.60×10-5)3 mol.K (4.50×10-6)3 W1=2.40×10-10J So,the work done on the bubble during the expansion is: W1=-2.40×10-10J. Process 2:Adiabatic collapse For an adiabatic process D?=P② B AY For a monatomic gas y=5/3 so, P2= 197品·3.60×10-5m5 (4.50×10-6m)5 c) P2=6.46×106Pa. And 及 nR T2= 6.46×106Pa专π·(4.50×10-6m)3 158×10-4 moles-8,31mdK d) T2 =18800 K.Lord,have mercy!That's hot! The work done by the bubble during an adiabatic process is W2=-△Einternal=-nC,△T Copyright C2011 American Association of Physics Teachers2011 Semifinal Exam Part A 4 a) n = 1.58 × 10−14 moles. Process 1: Isothermal expansion This process is isothermal, so T1 = T0 and P1V1 = nRT1 = nRT0 P1 = nRT0 V1 = 1.58 × 10−14 moles · 8.31 J mol·K · 293 K 4 3 π · (3.60 × 10−5 m)3 b) P1 = 197 N m2 = 197 Pa. The work done by the bubble is: W1 = nRT0 ln V1 V0 W1 = 1.58 × 10−14 moles · 8.31 J mol·K · 293 K · ln (3.60 × 10−5 ) 3 (4.50 × 10−6) 3 W1 = 2.40 × 10−10 J So, the work done on the bubble during the expansion is: W1 = −2.40 × 10−10 J. Process 2: Adiabatic collapse For an adiabatic process P1V γ 1 = P2V γ 2 P2 = P1V γ 1 V γ 2 For a monatomic gas γ = 5/3 so, P2 = 197 N m2 · (3.60 × 10−5 m)5 (4.50 × 10−6 m)5 c) P2 = 6.46 × 106 Pa. And T2 = P2V2 nR T2 = 6.46 × 106 Pa · 4 3 π · (4.50 × 10−6 m)3 1.58 × 10−14 moles · 8.31 J mol·K d) T2 = 18800 K. Lord, have mercy! That’s hot! The work done by the bubble during an adiabatic process is W2 = −∆Einternal = −nCv∆T Copyright c 2011 American Association of Physics Teachers