正在加载图片...
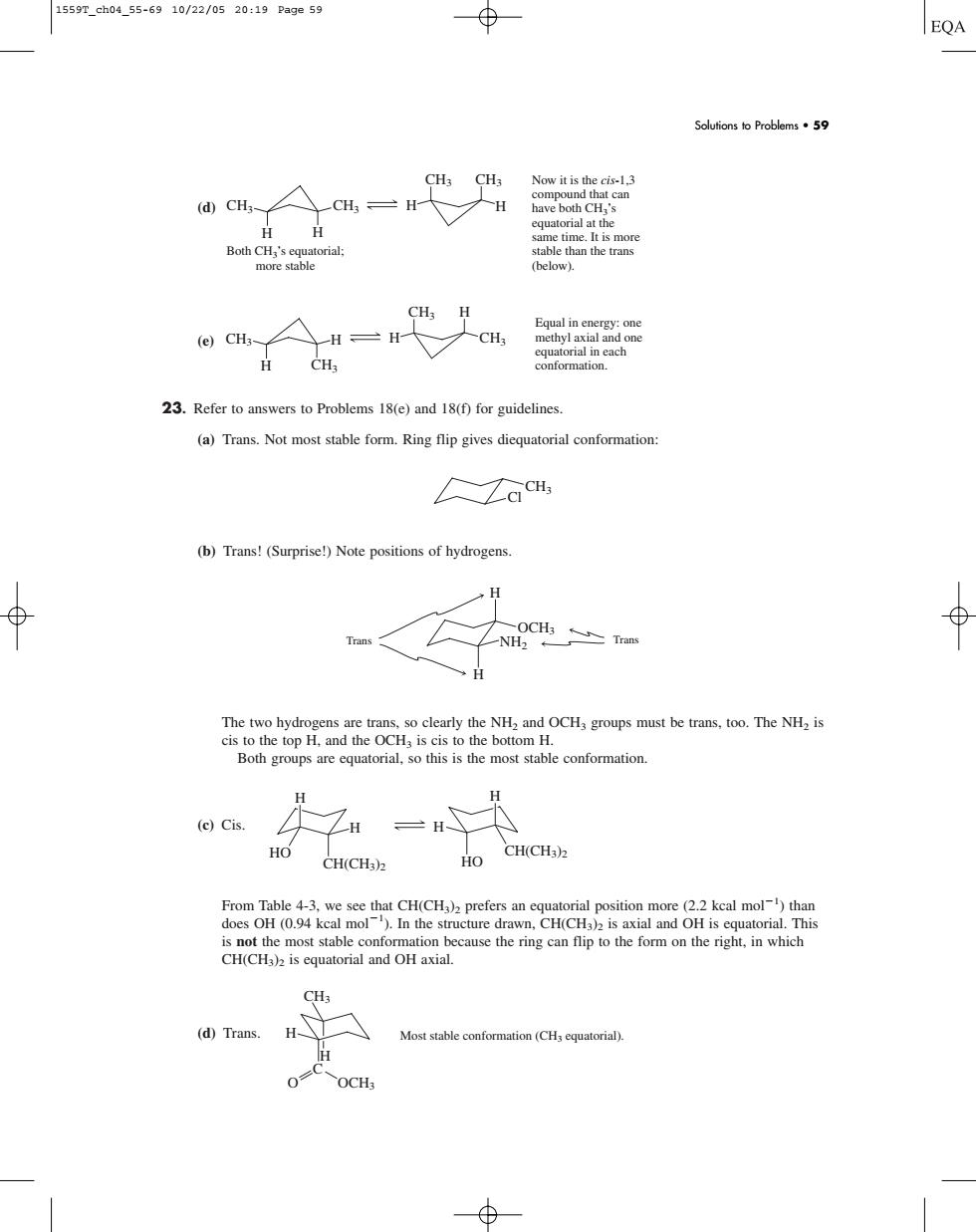
1559T_ch04_55-6910/22/0520:19Pa9e59 EQA Solufions o Problems5 @cH人cH,=H Lat the Both CH conformation. 23.Refer to answers to Problems 18(e)and 18(f)for guidelines. (a)Trans.Not most stable form.Ring flip gives diequatorial conformation: CiCH (b)Trans!(Surprise)Note positions of hydrogens H The two hydrogens are trans.so clearly the NH and OCH groups must be trans.too.The NH is H e)Cis. = HO CH(CHs) is not the most stable conformation because the ring can flip to the form on the right,in which CH(CH3)is equatorial and OH axia 1)Tran Most stable conformation(CH equatorial) OCH (d) (e) 23. Refer to answers to Problems 18(e) and 18(f) for guidelines. (a) Trans. Not most stable form. Ring flip gives diequatorial conformation: (b) Trans! (Surprise!) Note positions of hydrogens. The two hydrogens are trans, so clearly the NH2 and OCH3 groups must be trans, too. The NH2 is cis to the top H, and the OCH3 is cis to the bottom H. Both groups are equatorial, so this is the most stable conformation. (c) Cis. From Table 4-3, we see that CH(CH3)2 prefers an equatorial position more (2.2 kcal mol1 ) than does OH (0.94 kcal mol1 ). In the structure drawn, CH(CH3)2 is axial and OH is equatorial. This is not the most stable conformation because the ring can flip to the form on the right, in which CH(CH3)2 is equatorial and OH axial. (d) Trans. H H Most stable conformation (CH3 equatorial). CH3 O OCH3 C H H HO H HO H CH(CH3)2 CH(CH3)2 H H NH2 OCH3 Trans Trans Cl CH3 H H H H CH3 CH3 CH3 CH3 Equal in energy: one methyl axial and one equatorial in each conformation. H H H CH3 CH3 H CH3 CH3 Both CH3’s equatorial; more stable Now it is the cis-1,3 compound that can have both CH3’s equatorial at the same time. It is more stable than the trans (below). Solutions to Problems • 59 1559T_ch04_55-69 10/22/05 20:19 Page 59