正在加载图片...
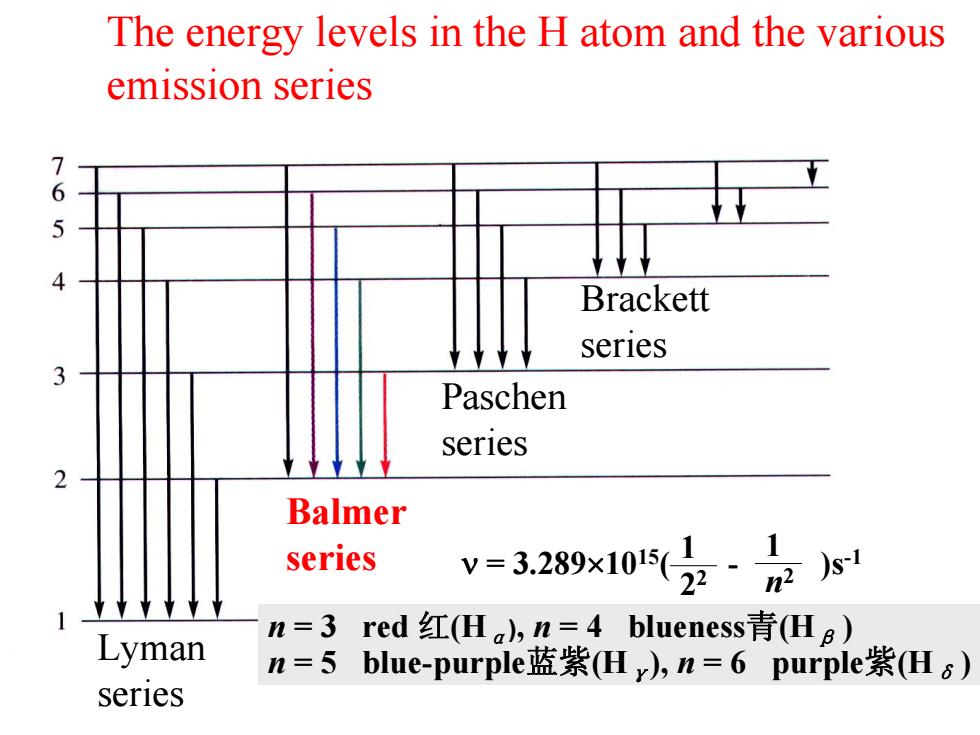
The energy levels in the H atom and the various emission series 76 Brackett series Paschen series Balmer series v=3.289x10z·s Lyman n=3red红(Ha,n=4 blueness青(Hg) n=5blue-purple蓝紫(Hy),n=6 purple紫(Hs) seriesThe energy levels in the H atom and the various emission series Balmer series Lyman series Paschen series Brackett series n = 3 red 红(H α), n = 4 blueness 青(H β ) n = 5 blue-purple蓝紫(H γ), n = 6 purple 紫(H δ ) ν = 3.289 ×1015( - )s 1 -1 2 2 1 n 2