正在加载图片...
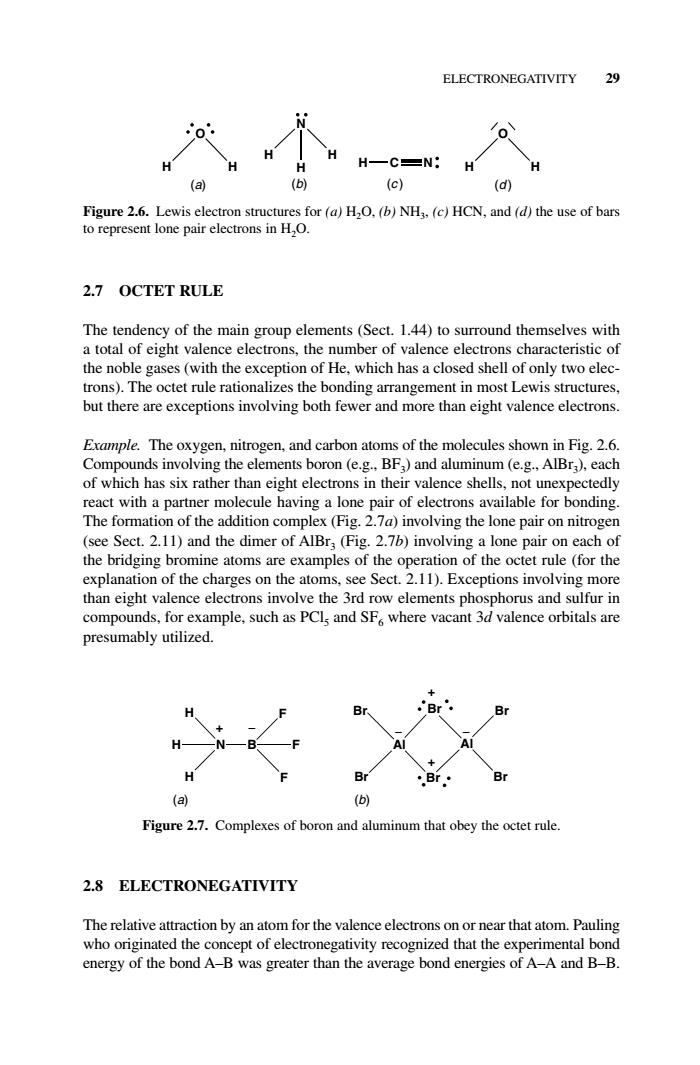
ELECTRONEGATIVITY 29 o H HC=N: H (a) (b) (c) (d) Figure 2.6.Lewis electron structures for(a)HO.(b)NH.(c)HCN.and (d)the use of bars to represent lone pair electrons in H,O. 2.7 OCTET RULE The tendency of the main group elements(Sect.1.44)to surround themselves with a total of eight valence electrons,the number of valence electrons characteristic of the noble gases (with the exception of He,which has a closed shell of only two elec- trons).The octet rule rationalizes the bonding arrangement in most Lewis structures but there are exceptions involving both fewer and m nore than eight valence electrons Example.The oxygen. nitrogen,and carbon atoms of the molecules shown in Fig.2.6 Compounds involving the elements boron (e.g..BF)and aluminum(e.g..AlBr).each of which has six rather than eight electrons in their valence shells,not unexpectedly react with a partner molecule having a lone pair of electrons available for bonding. The formation of the addition complex (Fig.2.7a)involving the lone pair on nitrogen (see Sect.2.11)and the dimer of AlBr:(Fig.2.7b)involving a lone pair on each of the bridging bromine atoms are examples of the operation of the octet rule(for the explanation of the charges on the at s.see Sect.2.11).Ex ceptions involvir than eigh nce ele the 3rd ro ement nd sulf for example,such as PCls and SF where vacant or in compoun lence orbitals are presumably utilized. (a) (b) Figure 2.7.Complexes of boron and aluminum that obey the octet rule 2.8 ELECTRONEGATIVITY The relative a action by an atom for the valence electrons onor near that atom.Pauling who ongir the concept of electronega d that the experiment energy of the bond A-B was greater than the average bond energies of A-A and B-B.2.7 OCTET RULE The tendency of the main group elements (Sect. 1.44) to surround themselves with a total of eight valence electrons, the number of valence electrons characteristic of the noble gases (with the exception of He, which has a closed shell of only two electrons). The octet rule rationalizes the bonding arrangement in most Lewis structures, but there are exceptions involving both fewer and more than eight valence electrons. Example. The oxygen, nitrogen, and carbon atoms of the molecules shown in Fig. 2.6. Compounds involving the elements boron (e.g., BF3) and aluminum (e.g., AlBr3), each of which has six rather than eight electrons in their valence shells, not unexpectedly react with a partner molecule having a lone pair of electrons available for bonding. The formation of the addition complex (Fig. 2.7a) involving the lone pair on nitrogen (see Sect. 2.11) and the dimer of AlBr3 (Fig. 2.7b) involving a lone pair on each of the bridging bromine atoms are examples of the operation of the octet rule (for the explanation of the charges on the atoms, see Sect. 2.11). Exceptions involving more than eight valence electrons involve the 3rd row elements phosphorus and sulfur in compounds, for example, such as PCl5 and SF6 where vacant 3d valence orbitals are presumably utilized. 2.8 ELECTRONEGATIVITY The relative attraction by an atom for the valence electrons on or near that atom. Pauling who originated the concept of electronegativity recognized that the experimental bond energy of the bond A–B was greater than the average bond energies of A–A and B–B. ELECTRONEGATIVITY 29 O H H N H H H H C N (d ) O H H (a) (b) (c ) Figure 2.6. Lewis electron structures for (a) H2O, (b) NH3, (c) HCN, and (d) the use of bars to represent lone pair electrons in H2O. N H H H B F F F Al Br Br Al Br Br Br Br (a) (b) + + + − − − Figure 2.7. Complexes of boron and aluminum that obey the octet rule. c02.qxd 5/17/2005 5:13 PM Page 29