正在加载图片...
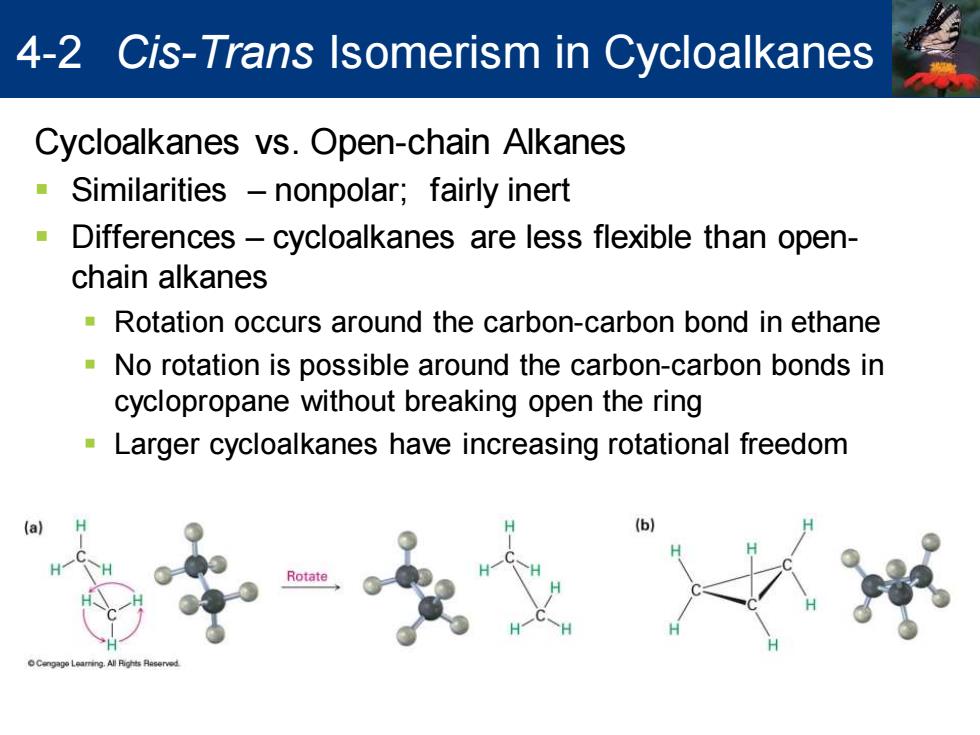
4-2 Cis-Trans Isomerism in Cycloalkanes Cycloalkanes vs.Open-chain Alkanes Similarities -nonpolar;fairly inert Differences-cycloalkanes are less flexible than open- chain alkanes Rotation occurs around the carbon-carbon bond in ethane No rotation is possible around the carbon-carbon bonds in cyclopropane without breaking open the ring Larger cycloalkanes have increasing rotational freedom (a) Rotate Cycloalkanes vs. Open-chain Alkanes ▪ Similarities – nonpolar; fairly inert ▪ Differences – cycloalkanes are less flexible than openchain alkanes ▪ Rotation occurs around the carbon-carbon bond in ethane ▪ No rotation is possible around the carbon-carbon bonds in cyclopropane without breaking open the ring ▪ Larger cycloalkanes have increasing rotational freedom 4-2 Cis-Trans Isomerism in Cycloalkanes