正在加载图片...
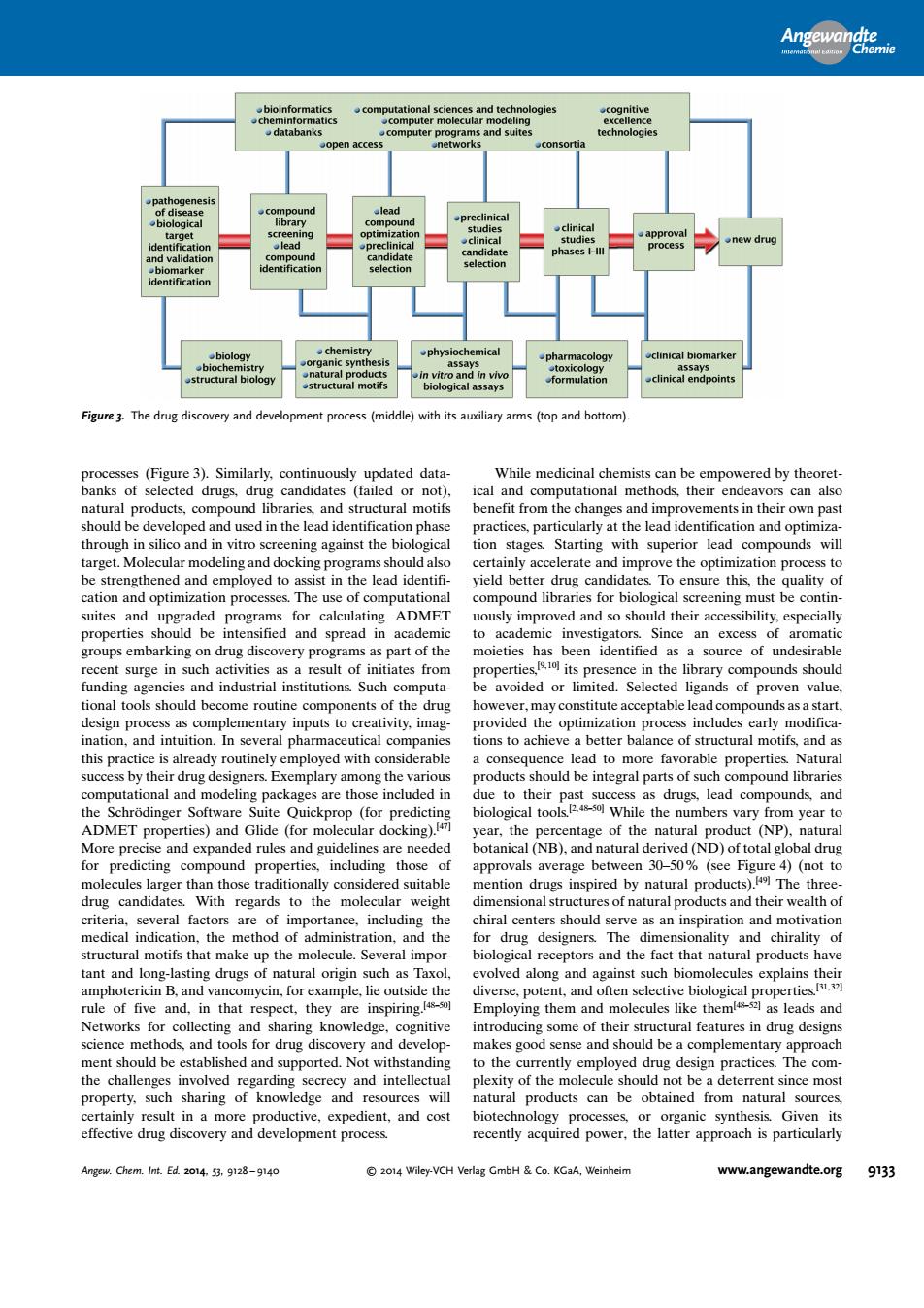
出 programs and cinical biomarke clinical endpoint Figure 3.The ry and deve ith ical and product ctural motif ents in the n silico and in vitro s ing against the idates To ensure this.the gu alit optim on process cening mus properties upd be intensified and spread in investigator an excess nds shoul avoided or limited. Selected alue pro ntary inputs to creativity imag ation cess includes early r ns to achleve ad products oukbeiniegmlpariofch ompound librarie the al to While the numbers a the nd es)an ded rules (NB). d (ND) uct including tho weight ac ral mot Several otors and the fact al nr ns thet e t five ha ience nd should be a complementary property.suc sharing of kno edge and will tural products can be from natural sources effective drug discovery and development process .Chem.Int.Ed H&Co.KGaA Wei andte.org 9133 processes (Figure 3). Similarly, continuously updated databanks of selected drugs, drug candidates (failed or not), natural products, compound libraries, and structural motifs should be developed and used in the lead identification phase through in silico and in vitro screening against the biological target. Molecular modeling and docking programs should also be strengthened and employed to assist in the lead identification and optimization processes. The use of computational suites and upgraded programs for calculating ADMET properties should be intensified and spread in academic groups embarking on drug discovery programs as part of the recent surge in such activities as a result of initiates from funding agencies and industrial institutions. Such computational tools should become routine components of the drug design process as complementary inputs to creativity, imagination, and intuition. In several pharmaceutical companies this practice is already routinely employed with considerable success by their drug designers. Exemplary among the various computational and modeling packages are those included in the Schrçdinger Software Suite Quickprop (for predicting ADMET properties) and Glide (for molecular docking).[47] More precise and expanded rules and guidelines are needed for predicting compound properties, including those of molecules larger than those traditionally considered suitable drug candidates. With regards to the molecular weight criteria, several factors are of importance, including the medical indication, the method of administration, and the structural motifs that make up the molecule. Several important and long-lasting drugs of natural origin such as Taxol, amphotericin B, and vancomycin, for example, lie outside the rule of five and, in that respect, they are inspiring.[48–50] Networks for collecting and sharing knowledge, cognitive science methods, and tools for drug discovery and development should be established and supported. Not withstanding the challenges involved regarding secrecy and intellectual property, such sharing of knowledge and resources will certainly result in a more productive, expedient, and cost effective drug discovery and development process. While medicinal chemists can be empowered by theoretical and computational methods, their endeavors can also benefit from the changes and improvements in their own past practices, particularly at the lead identification and optimization stages. Starting with superior lead compounds will certainly accelerate and improve the optimization process to yield better drug candidates. To ensure this, the quality of compound libraries for biological screening must be continuously improved and so should their accessibility, especially to academic investigators. Since an excess of aromatic moieties has been identified as a source of undesirable properties,[9, 10] its presence in the library compounds should be avoided or limited. Selected ligands of proven value, however, may constitute acceptable lead compounds as a start, provided the optimization process includes early modifications to achieve a better balance of structural motifs, and as a consequence lead to more favorable properties. Natural products should be integral parts of such compound libraries due to their past success as drugs, lead compounds, and biological tools.[2, 48–50] While the numbers vary from year to year, the percentage of the natural product (NP), natural botanical (NB), and natural derived (ND) of total global drug approvals average between 30–50% (see Figure 4) (not to mention drugs inspired by natural products).[49] The threedimensional structures of natural products and their wealth of chiral centers should serve as an inspiration and motivation for drug designers. The dimensionality and chirality of biological receptors and the fact that natural products have evolved along and against such biomolecules explains their diverse, potent, and often selective biological properties.[31, 32] Employing them and molecules like them[48–52] as leads and introducing some of their structural features in drug designs makes good sense and should be a complementary approach to the currently employed drug design practices. The complexity of the molecule should not be a deterrent since most natural products can be obtained from natural sources, biotechnology processes, or organic synthesis. Given its recently acquired power, the latter approach is particularly Figure 3. The drug discovery and development process (middle) with its auxiliary arms (top and bottom). Angewandte Chemie Angew. Chem. Int. Ed. 2014, 53, 9128 – 9140 2014 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim www.angewandte.org 9133�