正在加载图片...
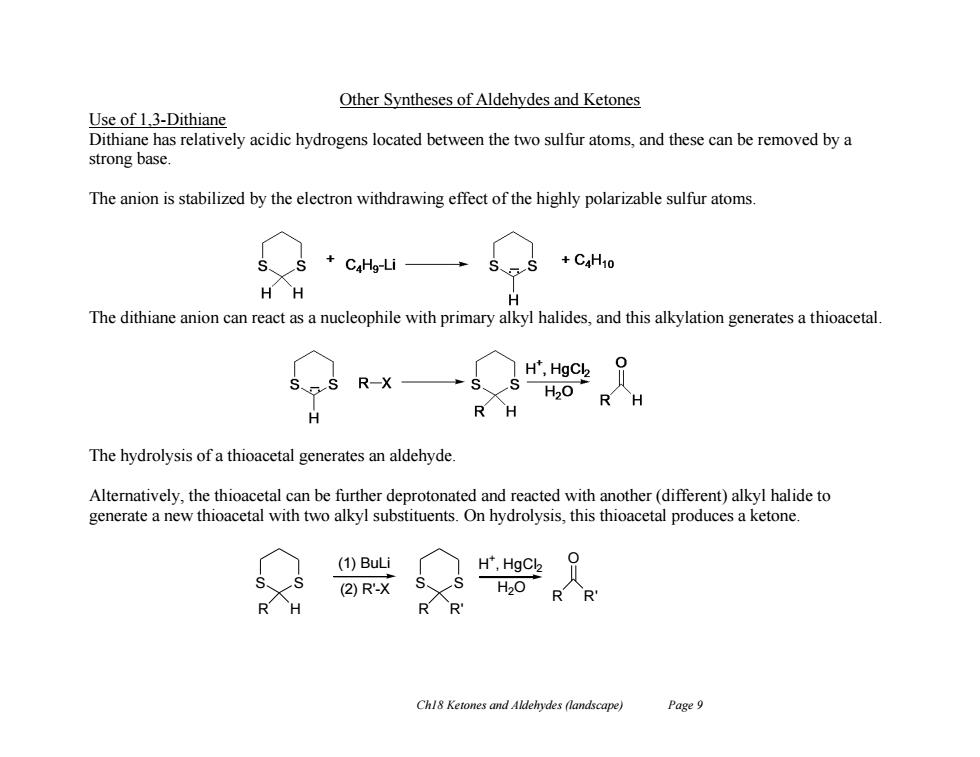
Other Syntheses of Aldehydes and Ketones Use of 1,3-Dithiane Dithiane has relatively acidic hydrogens located between the two sulfur atoms,and these can be removed by a strong base. The anion is stabilized by the electron withdrawing effect of the highly polarizable sulfur atoms. CaHg-Li +C4H10 H The dithiane anion can react as a nucleophile with primary alkyl halides,and this alkylation generates a thioacetal H",HgCl 0 R一X H20 R H The hydrolysis of a thioacetal generates an aldehyde. Alternatively,the thioacetal can be further deprotonated and reacted with another(different)alkyl halide to generate a new thioacetal with two alkyl substituents.On hydrolysis,this thioacetal produces a ketone. (1)BuLi H",HgCl2 (2)R-X H2O R R Chl8 Ketones and Aldehydes (landscape) Page 9 Ch18 Ketones and Aldehydes (landscape) Page 9 Other Syntheses of Aldehydes and Ketones Use of 1,3-Dithiane Dithiane has relatively acidic hydrogens located between the two sulfur atoms, and these can be removed by a strong base. The anion is stabilized by the electron withdrawing effect of the highly polarizable sulfur atoms. The dithiane anion can react as a nucleophile with primary alkyl halides, and this alkylation generates a thioacetal. The hydrolysis of a thioacetal generates an aldehyde. Alternatively, the thioacetal can be further deprotonated and reacted with another (different) alkyl halide to generate a new thioacetal with two alkyl substituents. On hydrolysis, this thioacetal produces a ketone. H + , HgCl2 H2O R R' (1) BuLi O (2) R'-X S S R H S S R R