正在加载图片...
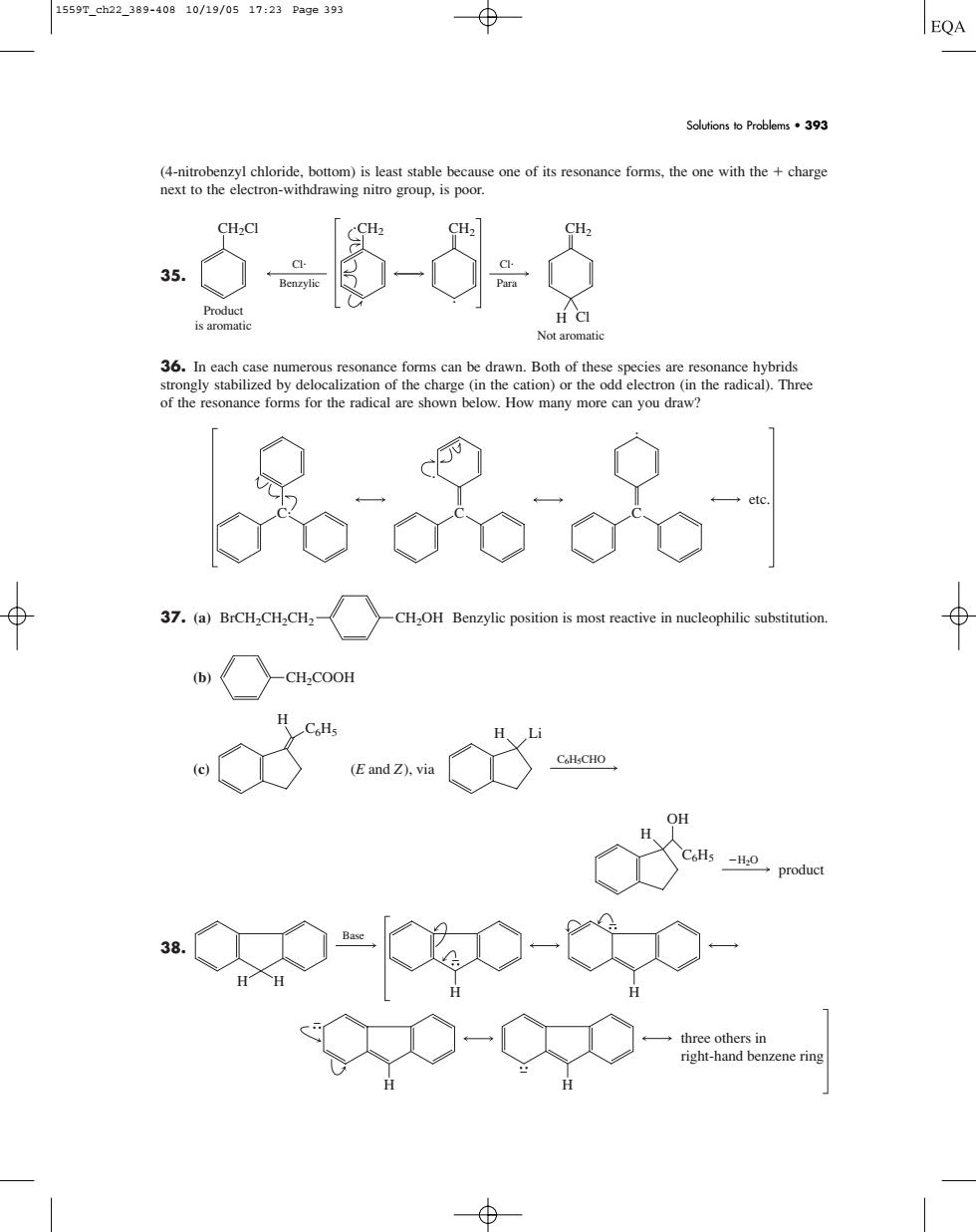
1559T_ch22_389-40810/19/0517:23Page39 EQA Soutionso Problems393 one of its r the one with the +charge Not aromatic 36.In each cas umerous re sonance forms can be drawn.Both of these species are resonance hybrid ation)or the od in the radical 品品只 37.(a)BrCH,CH,CH〈 CH2OH Benzylic position is most ive in nucleophilic substitutio 0o-0-0 (4-nitrobenzyl chloride, bottom) is least stable because one of its resonance forms, the one with the charge next to the electron-withdrawing nitro group, is poor. 35. 36. In each case numerous resonance forms can be drawn. Both of these species are resonance hybrids strongly stabilized by delocalization of the charge (in the cation) or the odd electron (in the radical). Three of the resonance forms for the radical are shown below. How many more can you draw? 37. (a) Benzylic position is most reactive in nucleophilic substitution. (b) (c) 38. H H three others in right-hand benzene ring H H H Base H H2O H OH C6H5 product H C6H5 C6H5CHO H Li (E and Z), via CH2COOH BrCH2CH2CH2 CH2OH C C etc. C CH2Cl CH2 CH2 Product is aromatic Benzylic Cl Cl Para CH2 H Cl Not aromatic Solutions to Problems • 393 1559T_ch22_389-408 10/19/05 17:23 Page 393������