正在加载图片...
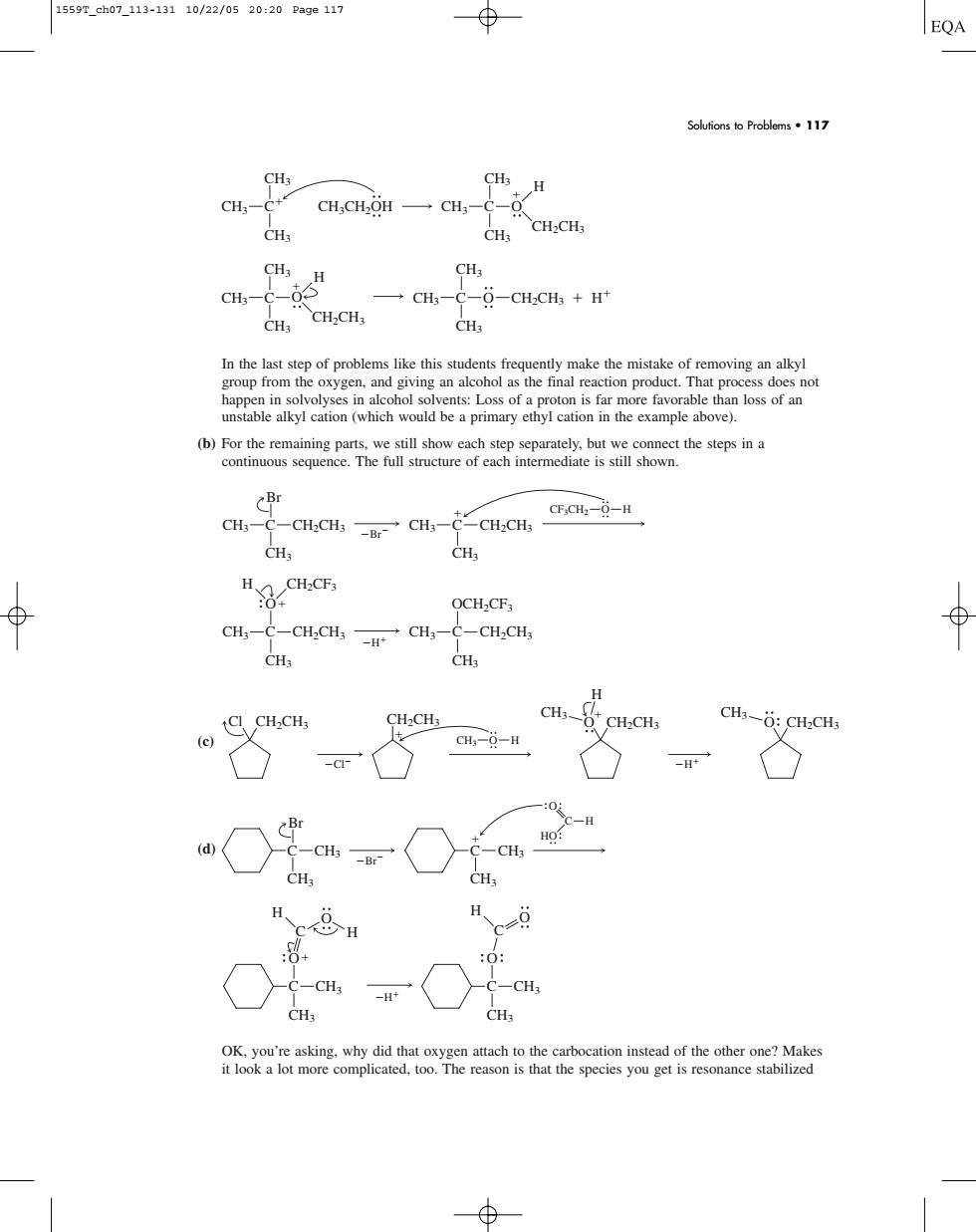
1559T_ch07-113-13110/22/0520:20Pa9e117 EQA Solutions to Problems.117 CH3-c+ CH.CH.OH CH,CH-CH →CH,-C--CHCH+H CHs CH.CHs CHa CBr CHC-CHCH,CH-CH,CH CH ⊕ OCH2CF3 CH-C-CH;CH,CH-C-CHCH, CH.CH CH3-:CH.CHs HO CH -CH In the last step of problems like this students frequently make the mistake of removing an alkyl group from the oxygen, and giving an alcohol as the final reaction product. That process does not happen in solvolyses in alcohol solvents: Loss of a proton is far more favorable than loss of an unstable alkyl cation (which would be a primary ethyl cation in the example above). (b) For the remaining parts, we still show each step separately, but we connect the steps in a continuous sequence. The full structure of each intermediate is still shown. (c) (d) OK, you’re asking, why did that oxygen attach to the carbocation instead of the other one? Makes it look a lot more complicated, too. The reason is that the species you get is resonance stabilized H C C CH3 CH3 O H H O C C CH3 CH3 O H O Br C CH3 C CH3 Br CH3 CH3 C H HO O H CH3 C CH3 OCH2CF3 CH3 C CH2CH3 CH3 CH2CH3 H CH2CF3 O Br CH3 C CH3 C CH2CH3 Br CH3 CH3 CH2CH3 CF3CH2 O H C CH3 CH3 CH3 CH2CH3 H H O C O CH3 CH3 CH3 CH2CH3 C CH3 CH3 CH3 CH2CH3 H C CH3 CH3 CH3CH2OH CH3 O Solutions to Problems • 117 Cl H Cl CH2CH3 CH2CH3 CH3 O H O CH2CH3 CH3 H O CH2CH3 CH3 1559T_ch07_113-131 10/22/05 20:20 Page 117��������������