正在加载图片...
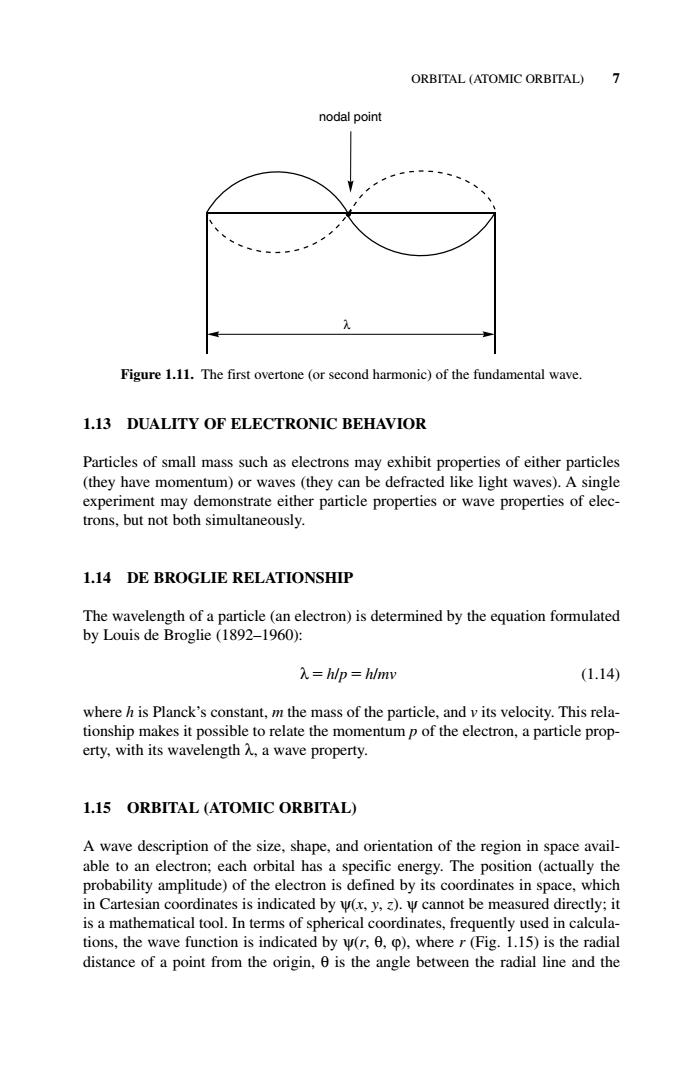
ORBITAL (ATOMIC ORBITAL)7 nodal point Figure 1.11.The first overtone (or second harmonic)of the fundamental wave. 1.13 DUALITY OF ELECTRONIC BEHAVIOR Particles of small mass such as may exhibit prop erties of either particles (they have momentum)or waves(they can be defracted like light waves).A single experiment may demonstrate either particle properties or wave properties of elec- trons,but not both simultaneously. 1.14 DE BROGLIE RELATIONSHIP The wavelengh()by by Louis de Broglie(1892-1960): 入=hlp=hlmw (1.14) where h is Planck's constant,m the mass of the particle,and v its velocity.This rela- tionship makes it possible to relate the momentump of the electron,a particle prop- erty,.with its wavelength,a wave property.. 1.15 ORBITAL (ATOMIC ORBITAL) A wave description of the size,shape,and orientation of the region in space avail- able to an electron;each orbital has a specific energy.The position (actually the probability amplitude)of the electron is defined by its coordinates in space,which in Cartesian coordinates is indicated by w(x,y,).W cannot be measured directly:it is a mathematical tool.In terms of spherical coordinates,frequently u sed in calcula tions,the wave function is indi ated h )where(Fig.1.5)is the radial distance ofa point from theangle between the radial lin and the1.13 DUALITY OF ELECTRONIC BEHAVIOR Particles of small mass such as electrons may exhibit properties of either particles (they have momentum) or waves (they can be defracted like light waves). A single experiment may demonstrate either particle properties or wave properties of electrons, but not both simultaneously. 1.14 DE BROGLIE RELATIONSHIP The wavelength of a particle (an electron) is determined by the equation formulated by Louis de Broglie (1892–1960): λ h/p h/mv (1.14) where h is Planck’s constant, m the mass of the particle, and v its velocity. This relationship makes it possible to relate the momentum p of the electron, a particle property, with its wavelength λ, a wave property. 1.15 ORBITAL (ATOMIC ORBITAL) A wave description of the size, shape, and orientation of the region in space available to an electron; each orbital has a specific energy. The position (actually the probability amplitude) of the electron is defined by its coordinates in space, which in Cartesian coordinates is indicated by ψ(x, y, z). ψ cannot be measured directly; it is a mathematical tool. In terms of spherical coordinates, frequently used in calculations, the wave function is indicated by ψ(r, θ, ϕ), where r (Fig. 1.15) is the radial distance of a point from the origin, θ is the angle between the radial line and the ORBITAL (ATOMIC ORBITAL) 7 nodal point λ Figure 1.11. The first overtone (or second harmonic) of the fundamental wave. c01.qxd 5/17/2005 5:12 PM Page 7