正在加载图片...
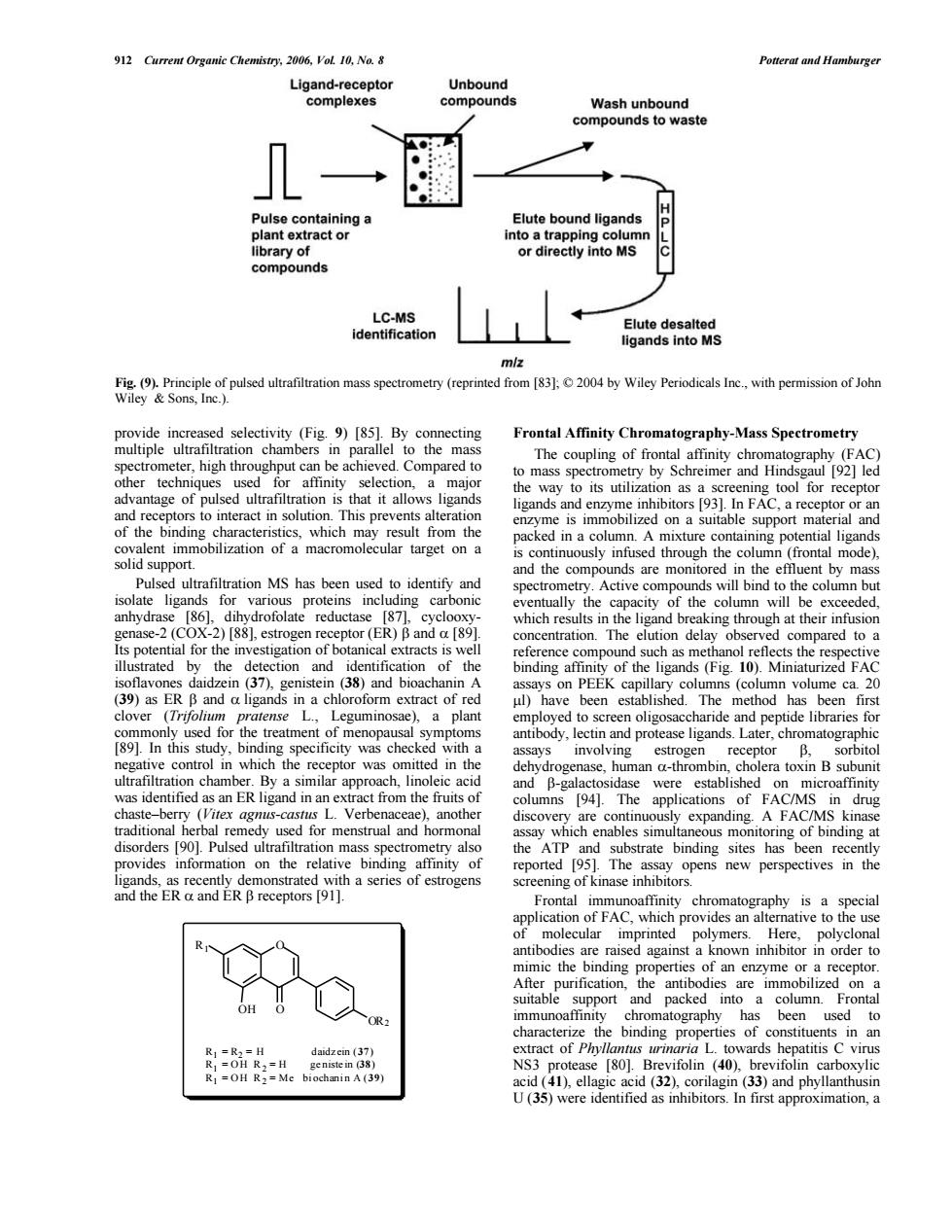
ua2omp8eor Elute bound ligands or directly into MS d LC-MS identification ctin Frontal affinity Chromatography-Mass spectrometry The co nling of frontal af nity ch AC to mass spectron 192]leo chaceo the tor or a ation spectro Active compo nds bind to theco bu refle (3()a anin 39)as4 nda ligand s in a chlorotorm ex ul)have The method has been 89.mscd which the wa in th human a-thrombin choler ton B ch 94. used fo and ho kina Pulsed u the ATP binding sites has e trated appl wn inhibit n orde ic the a has been use wards hep patitisC d (41).ella acid (3(40) (33)and p U(35)were identified as inhibitors.In first approximation,a 912 Current Organic Chemistry, 2006, Vol. 10, No. 8 Potterat and Hamburger provide increased selectivity (Fig. 9) [85]. By connecting multiple ultrafiltration chambers in parallel to the mass spectrometer, high throughput can be achieved. Compared to other techniques used for affinity selection, a major advantage of pulsed ultrafiltration is that it allows ligands and receptors to interact in solution. This prevents alteration of the binding characteristics, which may result from the covalent immobilization of a macromolecular target on a solid support. Pulsed ultrafiltration MS has been used to identify and isolate ligands for various proteins including carbonic anhydrase [86], dihydrofolate reductase [87], cyclooxygenase-2 (COX-2) [88], estrogen receptor (ER) b and a [89]. Its potential for the investigation of botanical extracts is well illustrated by the detection and identification of the isoflavones daidzein (37), genistein (38) and bioachanin A (39) as ER b and a ligands in a chloroform extract of red clover (Trifolium pratense L., Leguminosae), a plant commonly used for the treatment of menopausal symptoms [89]. In this study, binding specificity was checked with a negative control in which the receptor was omitted in the ultrafiltration chamber. By a similar approach, linoleic acid was identified as an ER ligand in an extract from the fruits of chaste–berry (Vitex agnus-castus L. Verbenaceae), another traditional herbal remedy used for menstrual and hormonal disorders [90]. Pulsed ultrafiltration mass spectrometry also provides information on the relative binding affinity of ligands, as recently demonstrated with a series of estrogens and the ER a and ER b receptors [91]. Frontal Affinity Chromatography-Mass Spectrometry The coupling of frontal affinity chromatography (FAC) to mass spectrometry by Schreimer and Hindsgaul [92] led the way to its utilization as a screening tool for receptor ligands and enzyme inhibitors [93]. In FAC, a receptor or an enzyme is immobilized on a suitable support material and packed in a column. A mixture containing potential ligands is continuously infused through the column (frontal mode), and the compounds are monitored in the effluent by mass spectrometry. Active compounds will bind to the column but eventually the capacity of the column will be exceeded, which results in the ligand breaking through at their infusion concentration. The elution delay observed compared to a reference compound such as methanol reflects the respective binding affinity of the ligands (Fig. 10). Miniaturized FAC assays on PEEK capillary columns (column volume ca. 20 ml) have been established. The method has been first employed to screen oligosaccharide and peptide libraries for antibody, lectin and protease ligands. Later, chromatographic assays involving estrogen receptor b, sorbitol dehydrogenase, human a-thrombin, cholera toxin B subunit and b-galactosidase were established on microaffinity columns [94]. The applications of FAC/MS in drug discovery are continuously expanding. A FAC/MS kinase assay which enables simultaneous monitoring of binding at the ATP and substrate binding sites has been recently reported [95]. The assay opens new perspectives in the screening of kinase inhibitors. Frontal immunoaffinity chromatography is a special application of FAC, which provides an alternative to the use of molecular imprinted polymers. Here, polyclonal antibodies are raised against a known inhibitor in order to mimic the binding properties of an enzyme or a receptor. After purification, the antibodies are immobilized on a suitable support and packed into a column. Frontal immunoaffinity chromatography has been used to characterize the binding properties of constituents in an extract of Phyllantus urinaria L. towards hepatitis C virus NS3 protease [80]. Brevifolin (40), brevifolin carboxylic acid (41), ellagic acid (32), corilagin (33) and phyllanthusin U (35) were identified as inhibitors. In first approximation, a Fig. (9). Principle of pulsed ultrafiltration mass spectrometry (reprinted from [83]; © 2004 by Wiley Periodicals Inc., with permission of John Wiley & Sons, Inc.). O OH O OR2 R1 R1 = R2 = H daidz ein (37) R1 = O H R 2 = H ge niste in (38) R1 = O H R 2 = Me bi ochani n A (39)