正在加载图片...
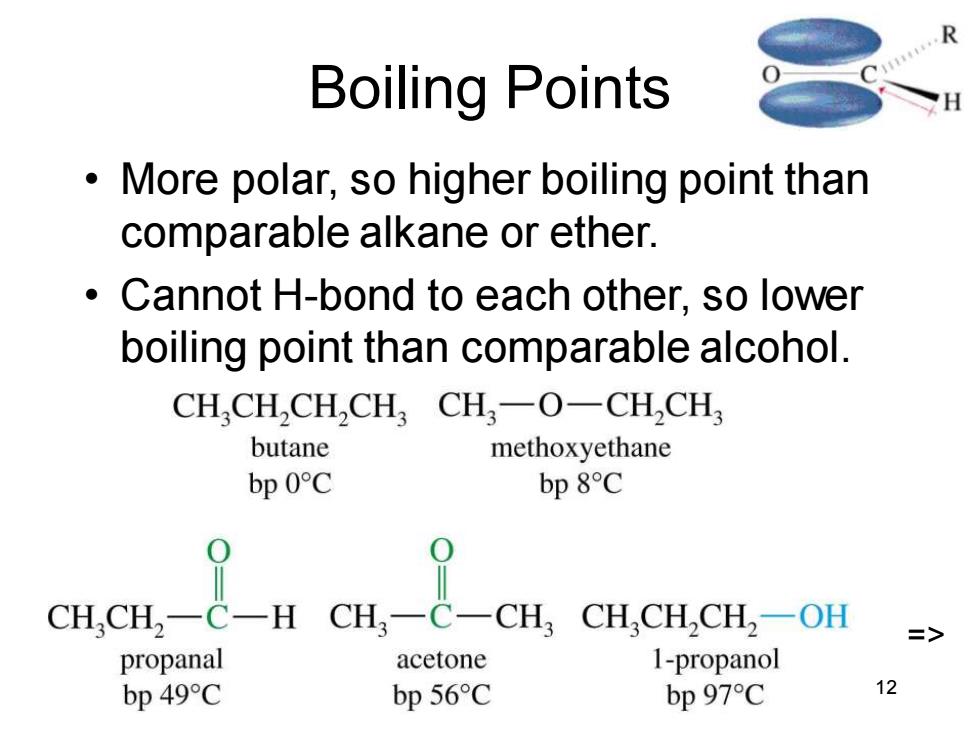
Boiling Points More polar,so higher boiling point than comparable alkane or ether. Cannot H-bond to each other,so lower boiling point than comparable alcohol. CH,CH,CH,CH3CH3一O一CH,CH butane methoxyethane bp 0C bp 8C CH,CH2-C一H CH,-C-CH,CH,CH,CH,-OH => propanal acetone 1-propanol bp 49C bp 56C bp 97C 12 Chapter 18 12 Boiling Points • More polar, so higher boiling point than comparable alkane or ether. • Cannot H-bond to each other, so lower boiling point than comparable alcohol. =>