正在加载图片...
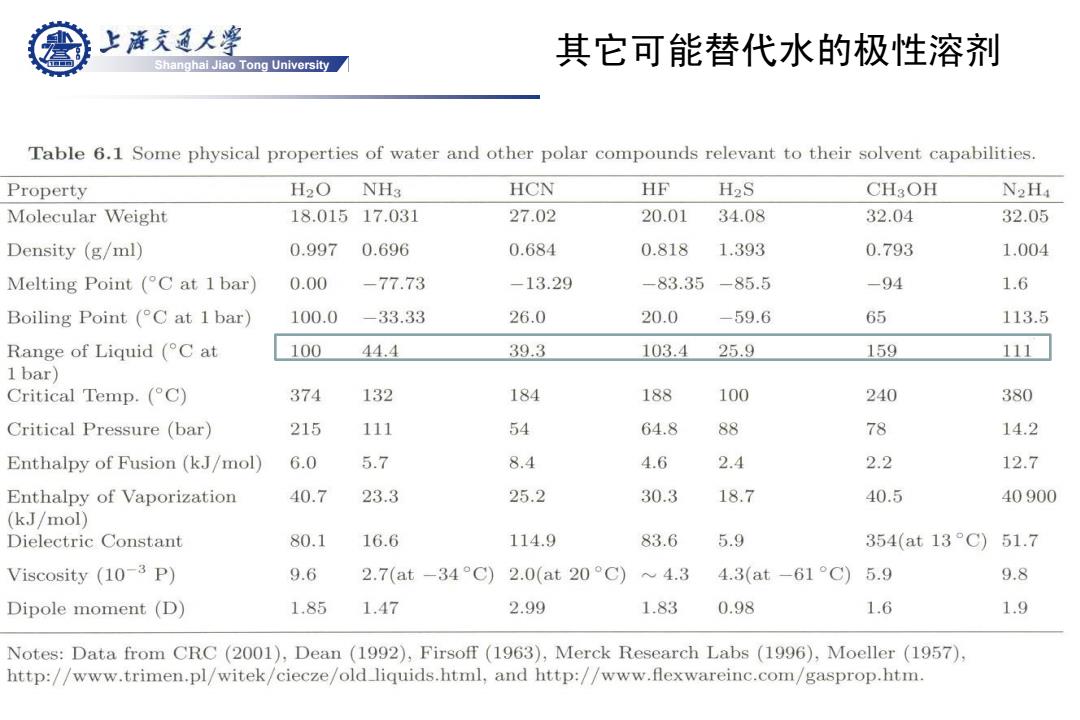
上游充通大¥ 其它可能替代水的极性溶剂 Shanghai Jiao Tong University Table 6.1 Some physical properties of water and other polar compounds relevant to their solvent capabilities. Property H2O NHa HCN HF H2S CH3OH N2H4 Molecular Weight 18.01517.031 27.02 20.01 34.08 32.04 32.05 Density (g/ml) 0.997 0.696 0.684 0.818 1.393 0.793 1.004 Melting Point (C at 1 bar) 0.00 -77.73 -13.29 -83.35-85.5 -94 1.6 Boiling Point (C at 1 bar) 100.0 -33.33 26.0 20.0 -59.6 65 113.5 Range of Liquid(°Cat 100 44.4 39.3 103.4 25.9 159 111 1bar) Critical Temp.(C) 374 132 184 188 100 240 380 Critical Pressure (bar) 215 111 54 64.8 88 78 14.2 Enthalpy of Fusion(kJ/mol) 6.0 5.7 8.4 4.6 2.4 2.2 12.7 Enthalpy of Vaporization 40.7 23.3 25.2 30.3 18.7 40.5 40900 (kJ/mol) Dielectric Constant 80.1 16.6 114.9 83.6 5.9 354(at13°C) 51.7 Viscosity (10-3 P) 9.6 2.7(at-34C) 2.0(at20°C) 4.3 4.3(at-61°C)5.9 9.8 Dipole moment (D) 1.85 1.47 2.99 1.83 0.98 1.6 1.9 Notes:Data from CRC (2001),Dean (1992),Firsoff(1963),Merck Research Labs (1996),Moeller (1957). http://www.trimen.pl/witek/ciecze/old liquids.html,and http://www.flexwareinc.com/gasprop.htm.Shanghai Jiao Tong University 其它可能替代水的极性溶剂