正在加载图片...
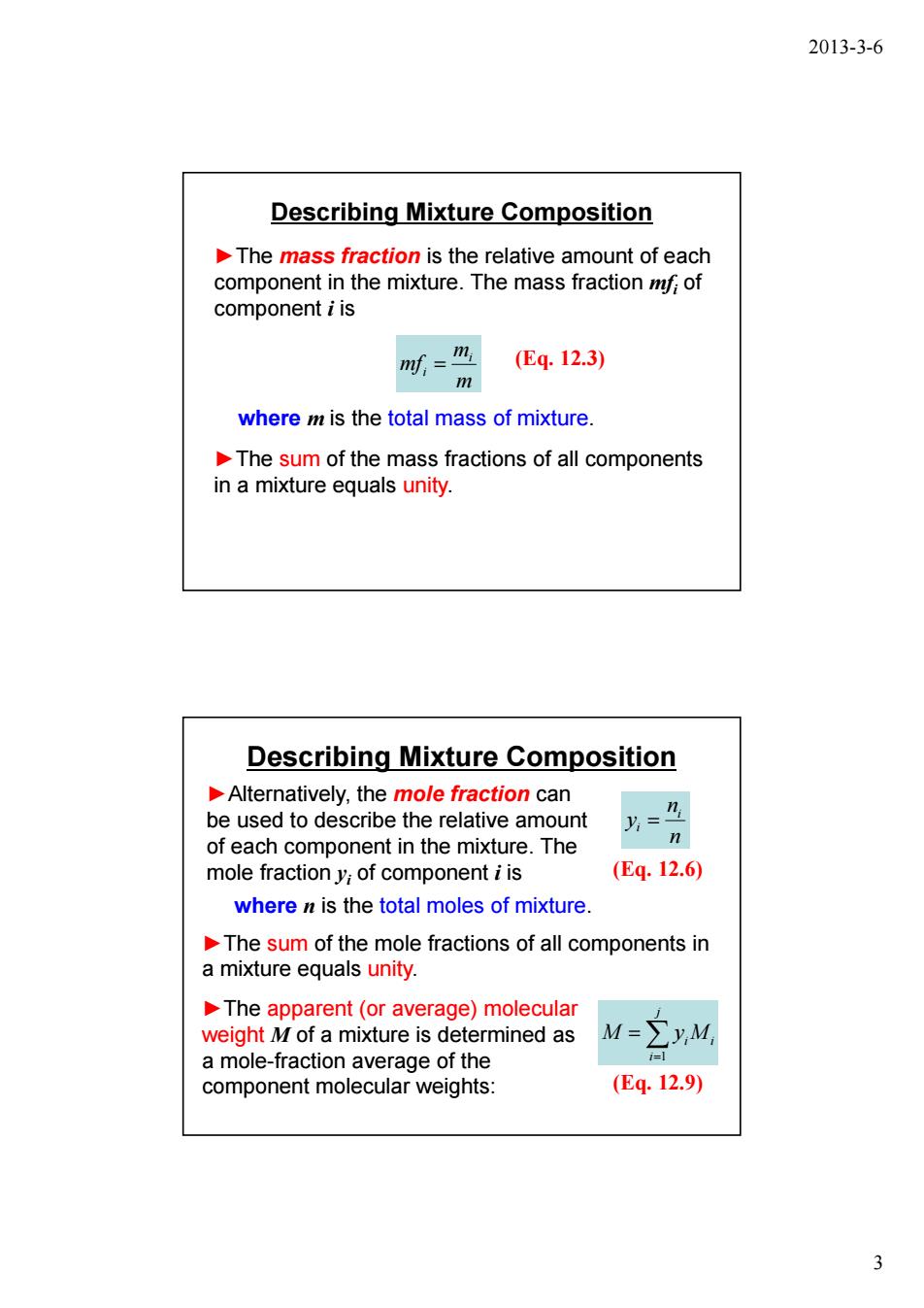
2013-3-6 Describing Mixture Composition The mass fraction is the relative amount of each component in the mixture.The mass fractionf;of component i is n时= (Eq.12.3) m where m is the total mass of mixture The sum of the mass fractions of all components in a mixture equals unity. Describing Mixture Composition Alternatively,the mole fraction can be used to describe the relative amount of each component in the mixture.The 丹 mole fractiony;of component i is (Eq.12.6) where n is the total moles of mixture The sum of the mole fractions of all components in a mixture equals unity. The apparent (or average)molecular weight M of a mixture is determined as M=yM a mole-fraction average of the 1 component molecular weights: (Eq.12.9) 3 2013-3-6 3 Describing Mixture Composition ►The mass fraction is the relative amount of each component in the mixture. The mass fraction mfi of component i is (Eq. 12.3) m m mf i i = ►The sum of the mass fractions of all components in a mixture equals unity. where m is the total mass of mixture. Describing Mixture Composition ►Alternatively, the mole fraction can be used to describe the relative amount of each component in the mixture. The mole fraction yi of component i is n n y i i = (Eq. 12.6) i j i M ∑ yi M = = 1 (Eq. 12.9) ►The sum of the mole fractions of all components in a mixture equals unity. ►The apparent (or average) molecular weight M of a mixture is determined as a mole-fraction average of the component molecular weights: where n is the total moles of mixture