正在加载图片...
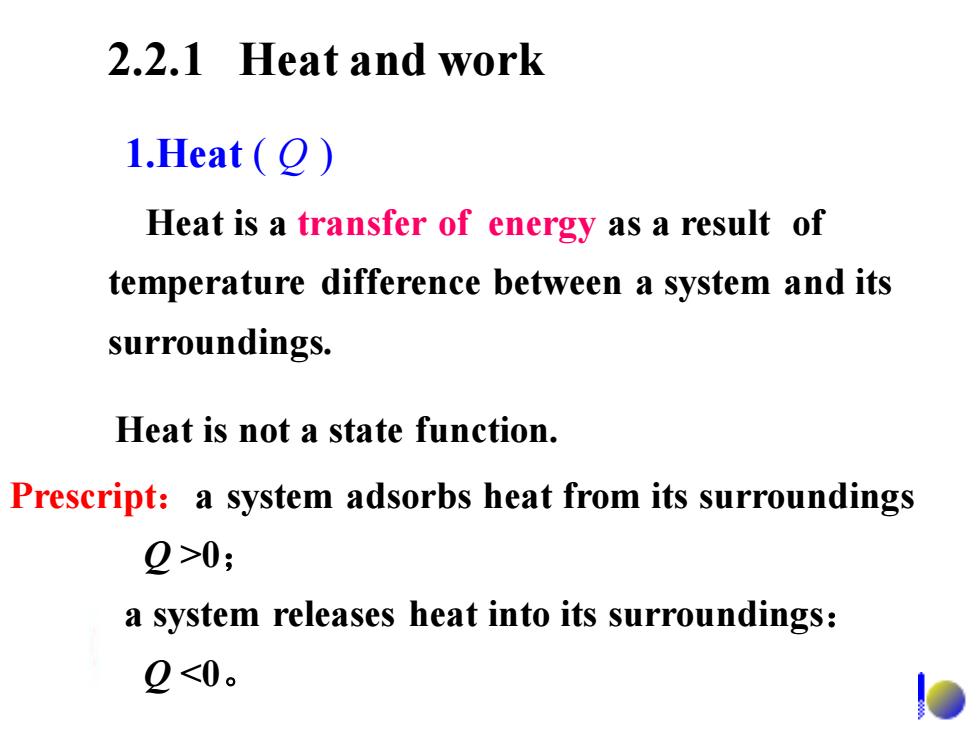
2.2.1 Heat and work 1.Heat (O) Heat is a transfer of energy as a result of temperature difference between a system and its surroundings. Heat is not a state function. Prescript:a system adsorbs heat from its surroundings 2>0; a system releases heat into its surroundings: 2<0。 2.2.1 Heat and work Heat is a transfer of energy as a result of temperature difference between a system and its surroundings. 1.Heat ( Q ) Heat is not a state function. Prescript:a system adsorbs heat from its surroundings Q >0; a system releases heat into its surroundings: Q <0