正在加载图片...
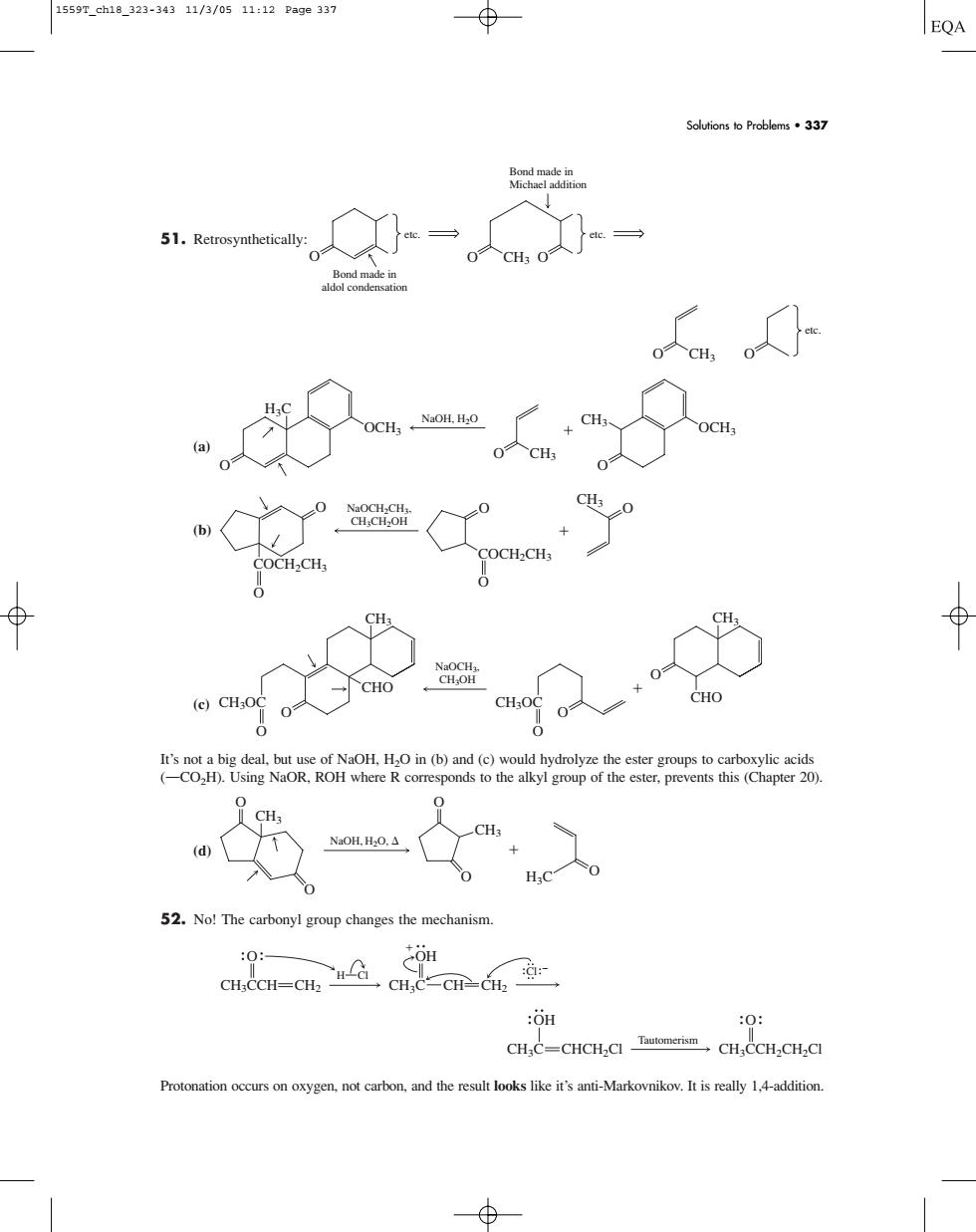
1559r.eh18323-34311/3/0511:12Page337 EQA Soutions to Problems337 51.Retrosynthetically CH OCH.CH H.C 52.No!The carbonyl group changes the mechanism. opor o :OH 0: CH,C-CHCH,.CCH,CH.C Protonation occurs on oxygen.not carbon,and the result looks like it's anti-Markovnikov.It is really 1,4-addition.51. Retrosynthetically: (a) (b) (c) It’s not a big deal, but use of NaOH, H2O in (b) and (c) would hydrolyze the ester groups to carboxylic acids (OCO2H). Using NaOR, ROH where R corresponds to the alkyl group of the ester, prevents this (Chapter 20). (d) 52. No! The carbonyl group changes the mechanism. Protonation occurs on oxygen, not carbon, and the result looks like it’s anti-Markovnikov. It is really 1,4-addition. O CH3CCH2CH2Cl Tautomerism OH CH3C CHCH2Cl OH CH3C CH CH2 O CH3CCH CH2 Cl H Cl O O O CH3 CH3 O H3C O NaOH, H2O, O O CH3OC CHO CH3 NaOCH3, CH3OH O O O CH3OC CHO CH3 O O O COCH2CH3 O COCH2CH3 CH3 NaOCH2CH3, O CH3CH2OH O CH3 OCH3 H3C NaOH, H2O O OCH3 CH3 O O CH3 etc. O O O CH3 etc. etc. O Bond made in aldol condensation Bond made in Michael addition Solutions to Problems • 337 1559T_ch18_323-343 11/3/05 11:12 Page 337