正在加载图片...
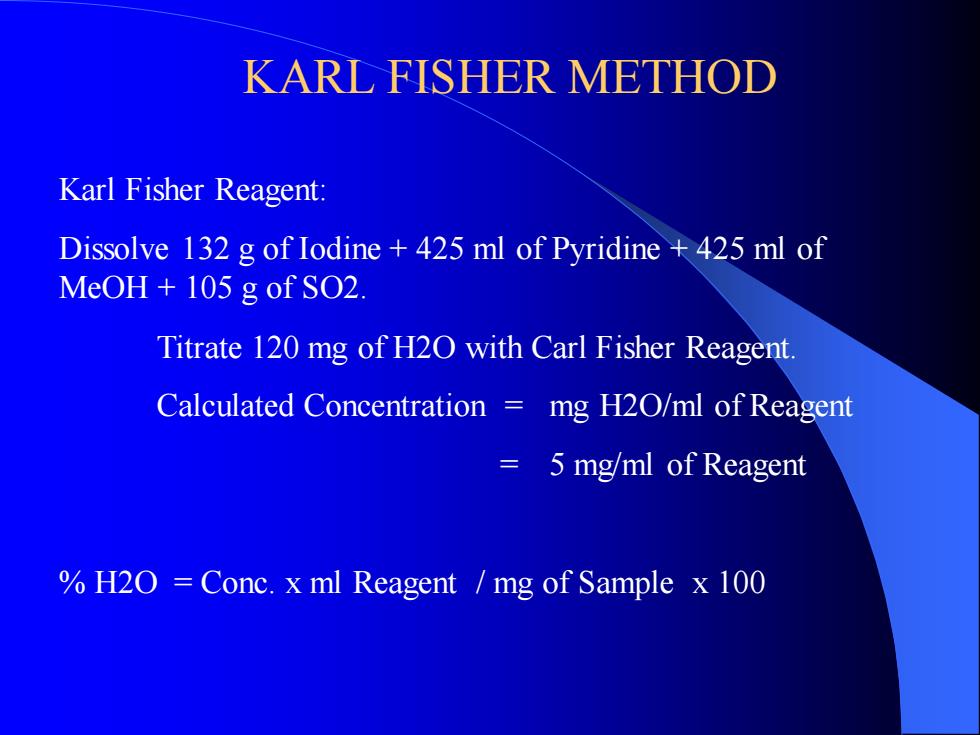
KARL FISHER METHOD Karl Fisher Reagent: Dissolve 132 g of Iodine + 425 ml of Pyridine + 425 ml of MeOH + 105 g of SO2. Titrate 120 mg of H2O with Carl Fisher Reagent. Calculated Concentration = mg H2O/ml of Reagent = 5 mg/ml of Reagent % H2O = Conc. x ml Reagent / mg of Sample x 100KARL FISHER METHOD Karl Fisher Reagent: Dissolve 132 g of Iodine + 425 ml of Pyridine + 425 ml of MeOH + 105 g of SO2. Titrate 120 mg of H2O with Carl Fisher Reagent. Calculated Concentration = mg H2O/ml of Reagent = 5 mg/ml of Reagent % H2O = Conc. x ml Reagent / mg of Sample x 100