正在加载图片...
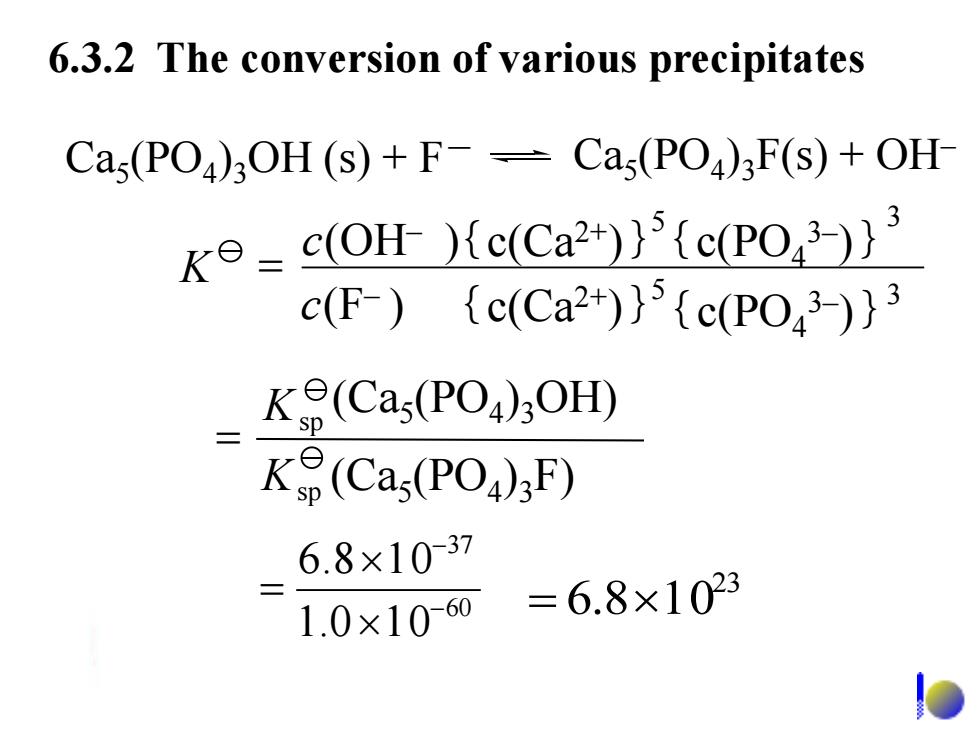
6.3.2 The conversion of various precipitates Cas(PO)3OH(s)+F-Cas(PO)F(s)+OH k=c(OH )(c(Ca2))5 (c(PO3))3 c(F){c(Ca2)}{c(Po43)}3 K(Cas(PO)OH) K(Cas(PO)F) 6.8×1037 1.0×1060 =6.8×1023 6.3.2 The conversion of various precipitates 60 37 1.0 1 0 6.8 1 0 - - = 23 = 6.810 (Ca5 (PO4 )3OH) (Ca5 (PO4 )3F) sp sp K K = Ca5 (PO4 )3OH (s) + F- Ca5 (PO4 )3F(s) + OH– (OH ) – c (F ) – = {c(Ca2+ c )} {c(Ca2+)} K 5 5 {c(PO4 3– )} 3 { 3 c(PO4 3– )}