正在加载图片...
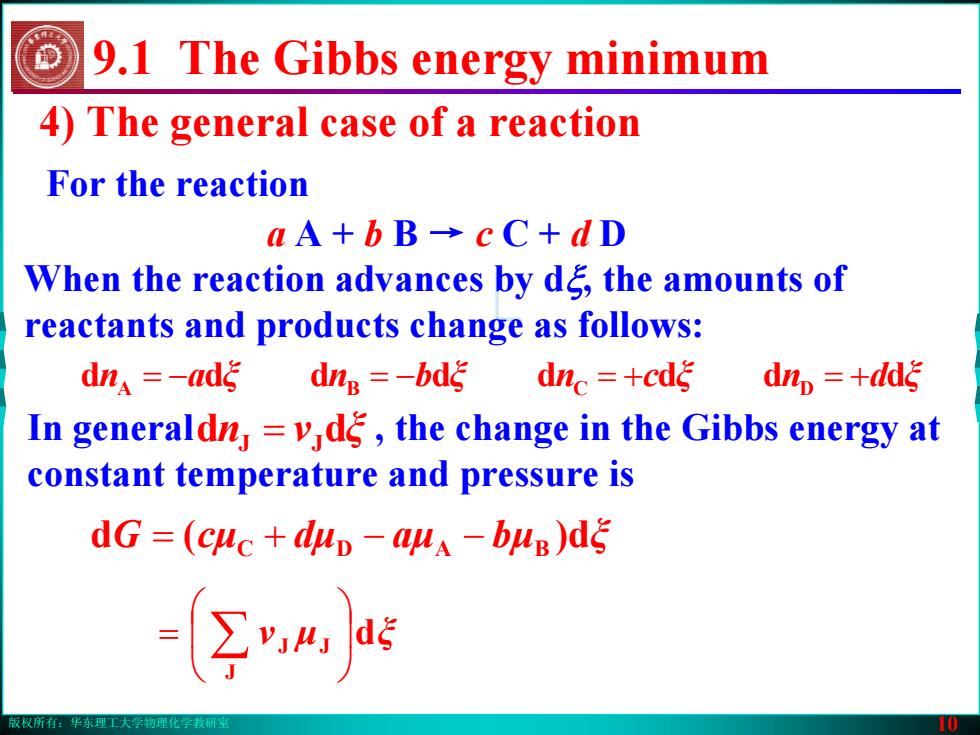
版权所有:华东理工大学物理化学教研室 10 4) The general case of a reaction For the reaction dξμν J JJ ⎟⎠⎞ ⎜⎝⎛ = ∑ a A + b B → c C + d D When the reaction advances by dξ, the amounts of reactants and products change as follows: an ξ bn ξ cn ξ dn dddddddd ξ A = − B = − C = + D = + In general , the change in the Gibbs energy at constant temperature and pressure is n dd ξν = JJ G (d cμ dμ aμ bμ d) ξ = C + D − − BA 9.1 The Gibbs energy minimum版权所有:华东理工大学物理化学教研室 10 4) The general case of a reaction For the reaction dξμν J JJ ⎟⎠⎞ ⎜⎝⎛ = ∑ a A + b B → c C + d D When the reaction advances by dξ, the amounts of reactants and products change as follows: an ξ bn ξ cn ξ dn dddddddd ξ A = − B = − C = + D = + In general , the change in the Gibbs energy at constant temperature and pressure is n dd ξν = JJ G (d cμ dμ aμ bμ d) ξ = C + D − − BA 9.1 The Gibbs energy minimum