正在加载图片...
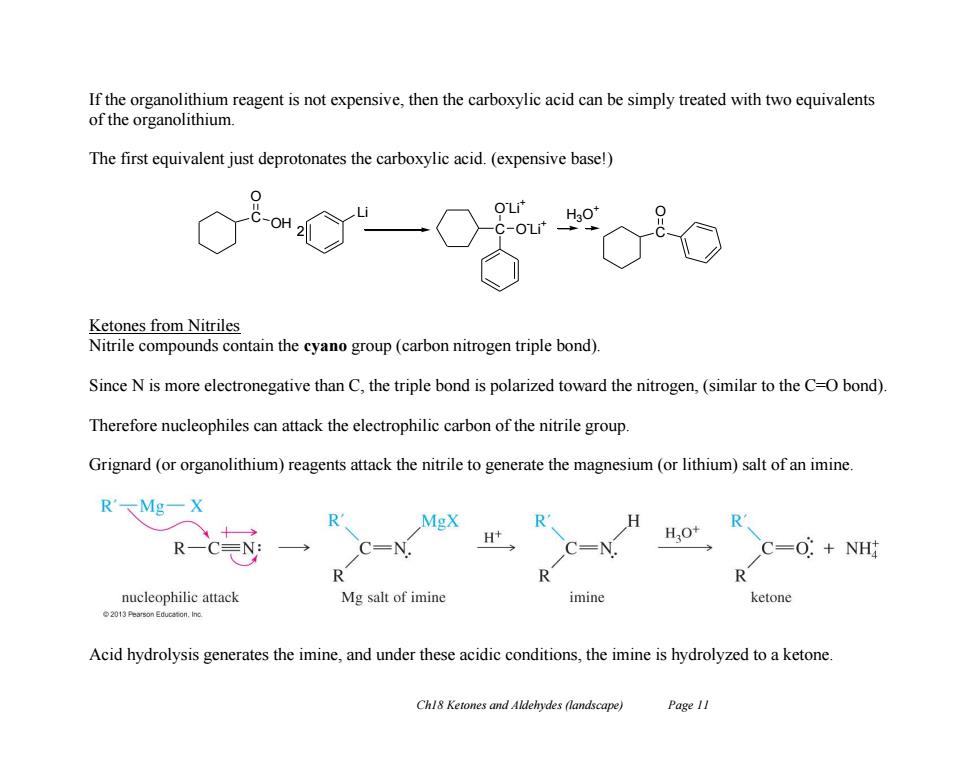
If the organolithium reagent is not expensive,then the carboxylic acid can be simply treated with two equivalents of the organolithium The first equivalent just deprotonates the carboxylic acid.(expensive base!) OH Ketones from Nitriles Nitrile compounds contain the cyano group(carbon nitrogen triple bond). Since N is more electronegative than C,the triple bond is polarized toward the nitrogen,(similar to the C-O bond) Therefore nucleophiles can attack the electrophilic carbon of the nitrile group. Grignard (or organolithium)reagents attack the nitrile to generate the magnesium(or lithium)salt of an imine. R'MgX R MgX R R H+ HO+ RC EN: C=0:NH R R nucleophilic attack Mg salt of imine imine ketone 2013 Pearson Educeton.Ino. Acid hydrolysis generates the imine,and under these acidic conditions,the imine is hydrolyzed to a ketone. Chl8 Ketones and Aldehydes (landscape) Page 11 Ch18 Ketones and Aldehydes (landscape) Page 11 If the organolithium reagent is not expensive, then the carboxylic acid can be simply treated with two equivalents of the organolithium. The first equivalent just deprotonates the carboxylic acid. (expensive base!) Ketones from Nitriles Nitrile compounds contain the cyano group (carbon nitrogen triple bond). Since N is more electronegative than C, the triple bond is polarized toward the nitrogen, (similar to the C=O bond). Therefore nucleophiles can attack the electrophilic carbon of the nitrile group. Grignard (or organolithium) reagents attack the nitrile to generate the magnesium (or lithium) salt of an imine. Acid hydrolysis generates the imine, and under these acidic conditions, the imine is hydrolyzed to a ketone. C OH O Li 2 C O - Li + O - Li + C H O 3O +