正在加载图片...
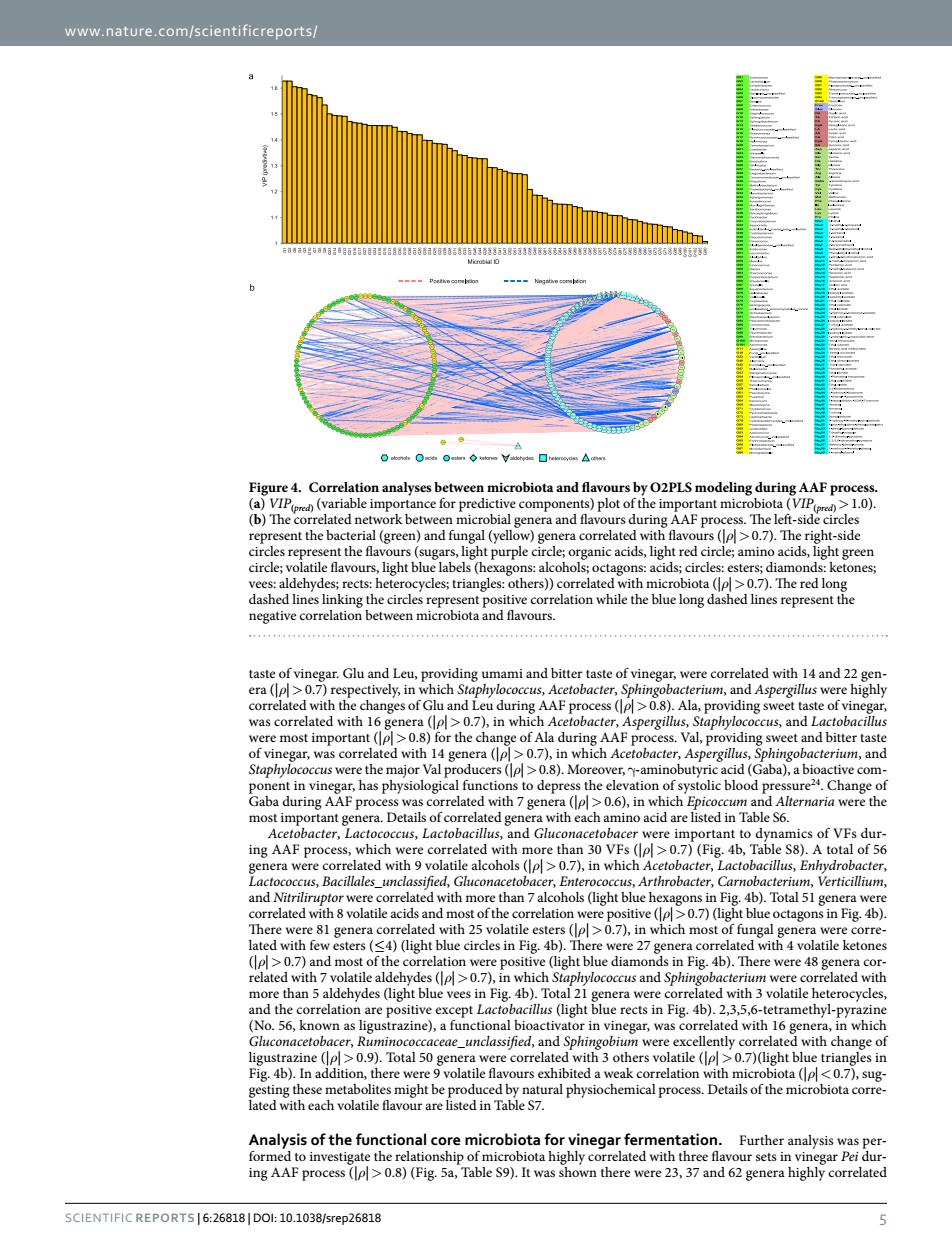
www.nature.com/scientificreports/ analyses betw microbiota and flavours by O2PLSm deling during AAF pro b)Th mpo bet AFD The le nt the lieht purple circle TtelogbeenmlCobiotandnavors with 4 and 11- acterium,and s to de /gene I with to cer,En Arthrobacter,Car elation w siti en ).n which st lated e g ine),a vith chan the micr ota cor Analysis of the fu onal co microbiota f th egar Pei dur D8 AAF 8(Fie 5a.Table S9)It ighly c SCIENTIFIC REPORTS6:26818DOl:10.1038/srep26818www.nature.com/scientificreports/ Scientific Reports | 6:26818 | DOI: 10.1038/srep26818 5 taste of vinegar. Glu and Leu, providing umami and bitter taste of vinegar, were correlated with 14 and 22 genera (|ρ|> 0.7) respectively, in which Staphylococcus, Acetobacter, Sphingobacterium, and Aspergillus were highly correlated with the changes of Glu and Leu during AAF process (|ρ|>0.8). Ala, providing sweet taste of vinegar, was correlated with 16 genera (|ρ|> 0.7), in which Acetobacter, Aspergillus, Staphylococcus, and Lactobacillus were most important (|ρ|> 0.8) for the change of Ala during AAF process. Val, providing sweet and bitter taste of vinegar, was correlated with 14 genera (|ρ|> 0.7), in which Acetobacter, Aspergillus, Sphingobacterium, and Staphylococcus were the major Val producers (|ρ|>0.8). Moreover, γ-aminobutyric acid (Gaba), a bioactive component in vinegar, has physiological functions to depress the elevation of systolic blood pressure24. Change of Gaba during AAF process was correlated with 7 genera (|ρ|> 0.6), in which Epicoccum and Alternaria were the most important genera. Details of correlated genera with each amino acid are listed in Table S6. Acetobacter, Lactococcus, Lactobacillus, and Gluconacetobacer were important to dynamics of VFs during AAF process, which were correlated with more than 30 VFs (|ρ| > 0.7) (Fig. 4b, Table S8). A total of 56 genera were correlated with 9 volatile alcohols (|ρ|> 0.7), in which Acetobacter, Lactobacillus, Enhydrobacter, Lactococcus, Bacillales_unclassified, Gluconacetobacer, Enterococcus, Arthrobacter, Carnobacterium, Verticillium, and Nitriliruptor were correlated with more than 7 alcohols (light blue hexagons in Fig. 4b). Total 51 genera were correlated with 8 volatile acids and most of the correlation were positive (|ρ|> 0.7) (light blue octagons in Fig. 4b). There were 81 genera correlated with 25 volatile esters (|ρ|> 0.7), in which most of fungal genera were correlated with few esters (≤4) (light blue circles in Fig. 4b). There were 27 genera correlated with 4 volatile ketones (|ρ|> 0.7) and most of the correlation were positive (light blue diamonds in Fig. 4b). There were 48 genera correlated with 7 volatile aldehydes (|ρ|> 0.7), in which Staphylococcus and Sphingobacterium were correlated with more than 5 aldehydes (light blue vees in Fig. 4b). Total 21 genera were correlated with 3 volatile heterocycles, and the correlation are positive except Lactobacillus (light blue rects in Fig. 4b). 2,3,5,6-tetramethyl-pyrazine (No. 56, known as ligustrazine), a functional bioactivator in vinegar, was correlated with 16 genera, in which Gluconacetobacer, Ruminococcaceae_unclassified, and Sphingobium were excellently correlated with change of ligustrazine (|ρ|> 0.9). Total 50 genera were correlated with 3 others volatile (|ρ|> 0.7)(light blue triangles in Fig. 4b). In addition, there were 9 volatile flavours exhibited a weak correlation with microbiota (|ρ|< 0.7), suggesting these metabolites might be produced by natural physiochemical process. Details of the microbiota correlated with each volatile flavour are listed in Table S7. Analysis of the functional core microbiota for vinegar fermentation. Further analysis was performed to investigate the relationship of microbiota highly correlated with three flavour sets in vinegar Pei during AAF process (|ρ|> 0.8) (Fig. 5a, Table S9). It was shown there were 23, 37 and 62 genera highly correlated Figure 4. Correlation analyses between microbiota and flavours by O2PLS modeling during AAF process. (a) VIP(pred) (variable importance for predictive components) plot of the important microbiota (VIP(pred)>1.0). (b) The correlated network between microbial genera and flavours during AAF process. The left-side circles represent the bacterial (green) and fungal (yellow) genera correlated with flavours (|ρ|>0.7). The right-side circles represent the flavours (sugars, light purple circle; organic acids, light red circle; amino acids, light green circle; volatile flavours, light blue labels (hexagons: alcohols; octagons: acids; circles: esters; diamonds: ketones; vees: aldehydes; rects: heterocycles; triangles: others)) correlated with microbiota (|ρ|>0.7). The red long dashed lines linking the circles represent positive correlation while the blue long dashed lines represent the negative correlation between microbiota and flavours