正在加载图片...
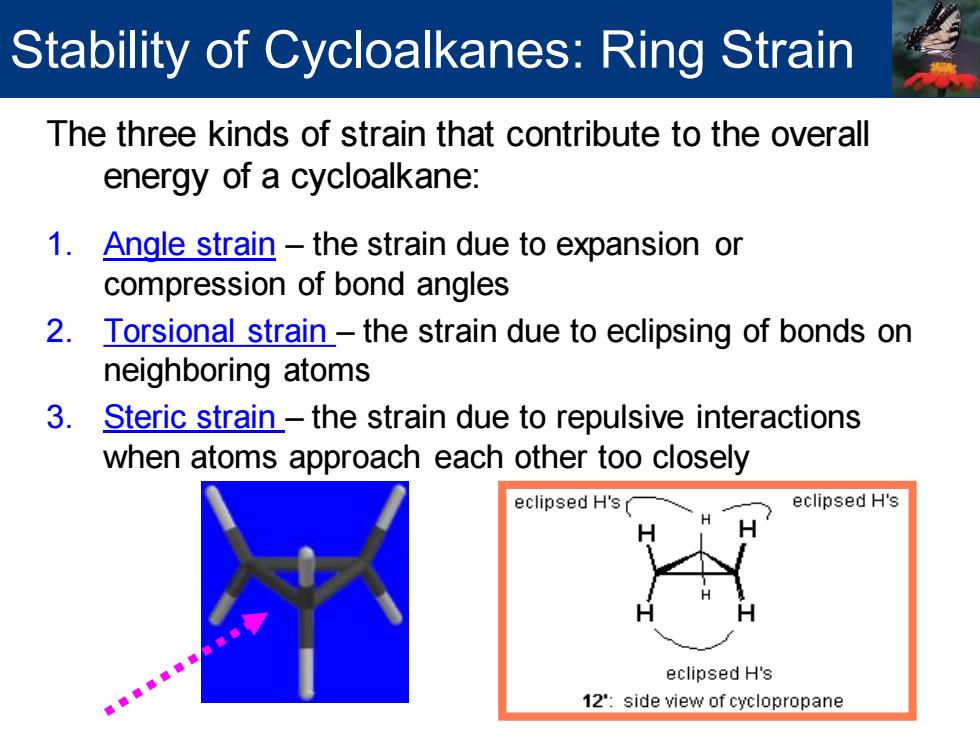
Stability of Cycloalkanes:Ring S Strain The three kinds of strain that contribute to the overall energy of a cycloalkane: 1.Angle strain-the strain due to expansion or compression of bond angles 2.Torsional strain-the strain due to eclipsing of bonds on neighboring atoms 3. Steric strain-the strain due to repulsive interactions when atoms approach each other too closely eclipsed H's eclipsed H's eclipsed H's 12:side view of cyclopropaneThe three kinds of strain that contribute to the overall energy of a cycloalkane: 1. Angle strain – the strain due to expansion or compression of bond angles 2. Torsional strain – the strain due to eclipsing of bonds on neighboring atoms 3. Steric strain – the strain due to repulsive interactions when atoms approach each other too closely Stability of Cycloalkanes: Ring Strain