正在加载图片...
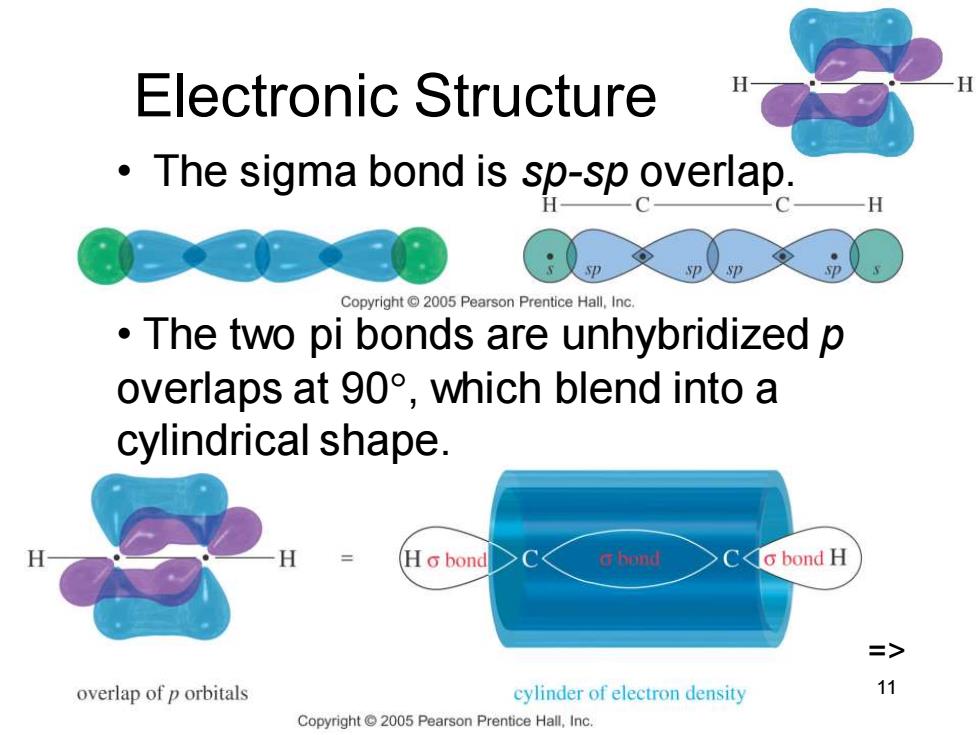
Electronic Structure The sigma bond is sp-sp overlap. Copyright2005 Pearson Prentice Hall.Inc The two pi bonds are unhybridized p overlaps at90°,which blend into a cylindrical shape. H Ho bond G hond Co bond H => overlap of p orbitals cylinder of electron density 11 Copyright 2005 Pearson Prentice Hall,Inc.Chapter 9 11 Electronic Structure • The sigma bond is sp-sp overlap. • The two pi bonds are unhybridized p overlaps at 90, which blend into a cylindrical shape. =>