正在加载图片...
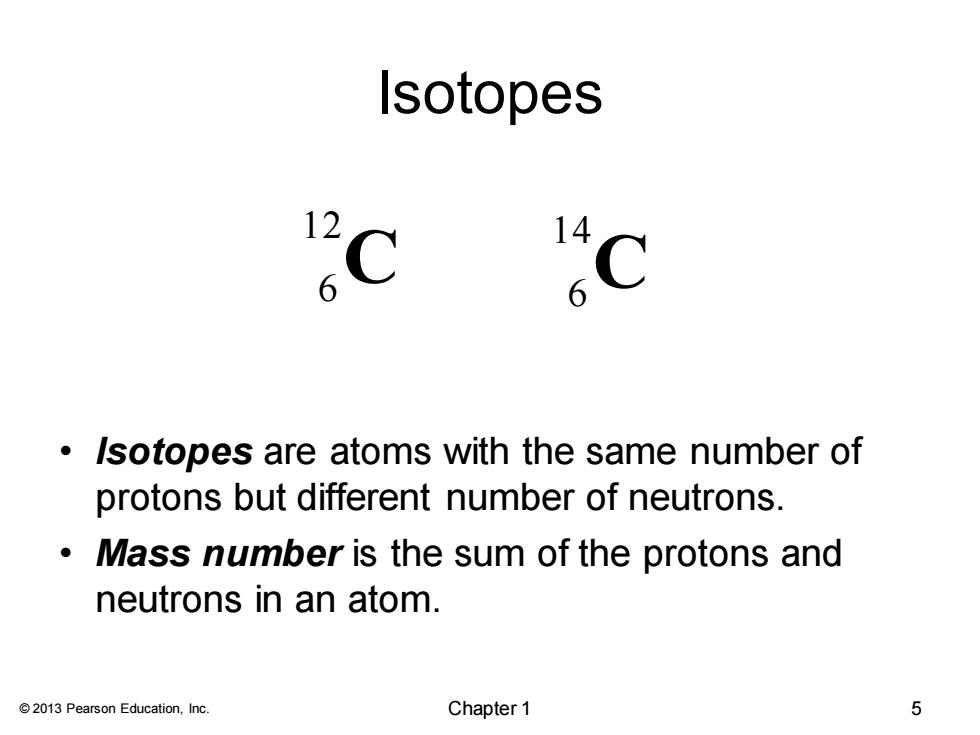
Isotopes 2 6 Isotopes are atoms with the same number of protons but different number of neutrons. Mass number is the sum of the protons and neutrons in an atom. 2013 Pearson Education,Inc. Chapter 1 5© 2013 Pearson Education, Inc. Isotopes • Isotopes are atoms with the same number of protons but different number of neutrons. • Mass number is the sum of the protons and neutrons in an atom. C 1 2 6 6 C 1 4 Chapter 1 5