正在加载图片...
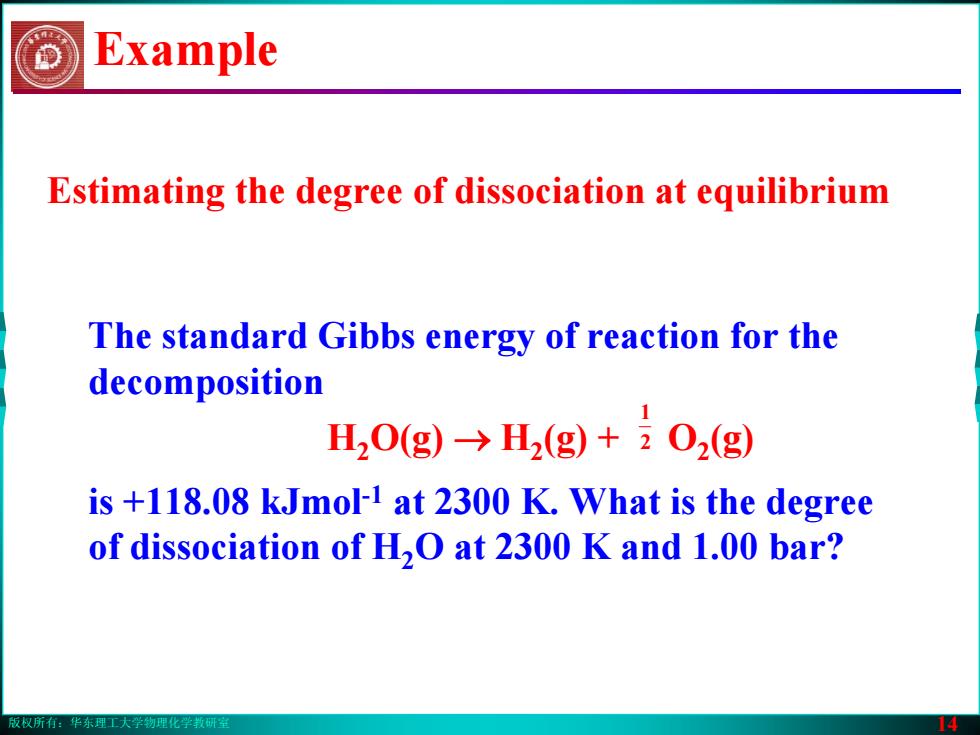
版权所有:华东理工大学物理化学教研室 14 Example The standard Gibbs energy of reaction for the decomposition H2O(g) → H2(g) + O2(g) is +118.08 kJmol-1 at 2300 K. What is the degree of dissociation of H2O at 2300 K and 1.00 bar? 2 1 Estimating the degree of dissociation at equilibrium版权所有:华东理工大学物理化学教研室 14 Example The standard Gibbs energy of reaction for the decomposition H2O(g) → H2(g) + O2(g) is +118.08 kJmol-1 at 2300 K. What is the degree of dissociation of H2O at 2300 K and 1.00 bar? 2 1 Estimating the degree of dissociation at equilibrium