正在加载图片...
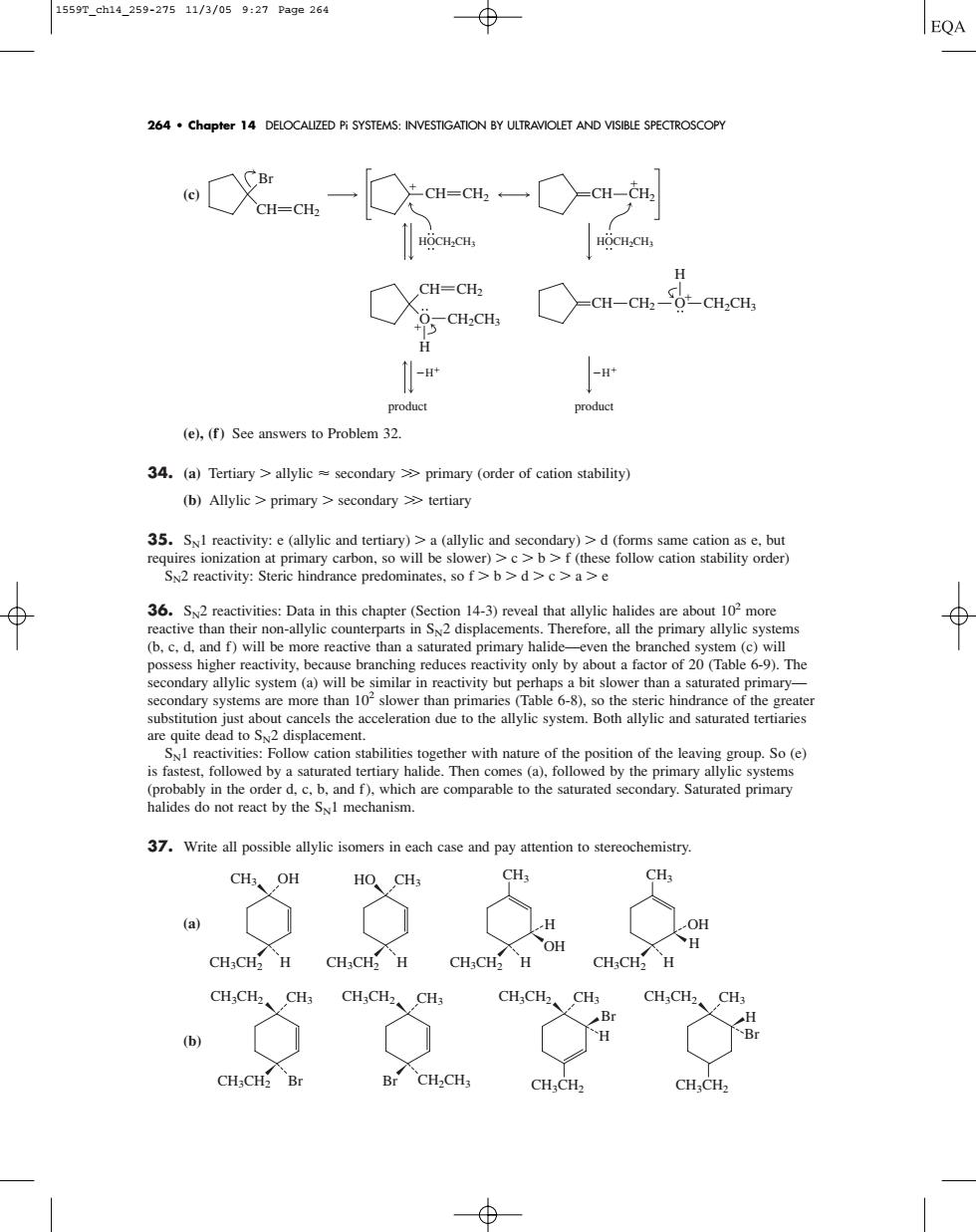
15597.eh14259-27511/3/059:27Pag0264 EQA 264.chapter 14 DELOCALIZED Pi SYSTEMS:INVESTIGATON BY ULTRAVIOLET AND VISIBLE SPECTROSCOPY HOCH.CH ○a-at-6-aa, -w product product (e),(f)See answers to Problem32. 34.(a)Tertiary>(order of cation stability (b)Allylic>primary>secondary tertiary S2 reactivity:Steric hindrance predominates. w cation branching reduces reactivity only by 6-9).The secondary systems are more than 0slower than primaries Table 6-8).so the steric hindrance of the cation stabilities together with nature of the position of the eavin(e) 37.Write all possible allylic isomers in each case and pay attention to stereochemistry. CH、OH HO CH3 CH3 CHICH H CHCH H CHICH H OH CH:CH H CHCH、CHa、OH CH:CH Br BCH-CHs CH;CH2 CH:CH,(c) (e), (f) See answers to Problem 32. 34. (a) Tertiary allylic secondary primary (order of cation stability) (b) Allylic primary secondary tertiary 35. SN1 reactivity: e (allylic and tertiary) a (allylic and secondary) d (forms same cation as e, but requires ionization at primary carbon, so will be slower) c b f (these follow cation stability order) SN2 reactivity: Steric hindrance predominates, so f b d c a e 36. SN2 reactivities: Data in this chapter (Section 14-3) reveal that allylic halides are about 102 more reactive than their non-allylic counterparts in SN2 displacements. Therefore, all the primary allylic systems (b, c, d, and f) will be more reactive than a saturated primary halide—even the branched system (c) will possess higher reactivity, because branching reduces reactivity only by about a factor of 20 (Table 6-9). The secondary allylic system (a) will be similar in reactivity but perhaps a bit slower than a saturated primary— secondary systems are more than 102 slower than primaries (Table 6-8), so the steric hindrance of the greater substitution just about cancels the acceleration due to the allylic system. Both allylic and saturated tertiaries are quite dead to SN2 displacement. SN1 reactivities: Follow cation stabilities together with nature of the position of the leaving group. So (e) is fastest, followed by a saturated tertiary halide. Then comes (a), followed by the primary allylic systems (probably in the order d, c, b, and f), which are comparable to the saturated secondary. Saturated primary halides do not react by the SN1 mechanism. 37. Write all possible allylic isomers in each case and pay attention to stereochemistry. (a) (b) CH3CH2 CH3 CH3CH2 CH3CH2 Br CH3 CH3CH2 CH3 CH3CH2 CH3 Br CH2CH3 H CH3CH2 CH3CH2 Br H Br CH3 OH CH3CH2 H HO CH3 CH3CH2 H CH3 CH3 OH H CH3CH2 H H OH CH3CH2 H product product CH HOCH2CH3 Br CH2 CH CH2 CH CH2 H H CH CH2 CH O O H H CH2 CH2CH3 CH2CH3 HOCH2CH3 264 • Chapter 14 DELOCALIZED Pi SYSTEMS: INVESTIGATION BY ULTRAVIOLET AND VISIBLE SPECTROSCOPY 1559T_ch14_259-275 11/3/05 9:27 Page 264