正在加载图片...
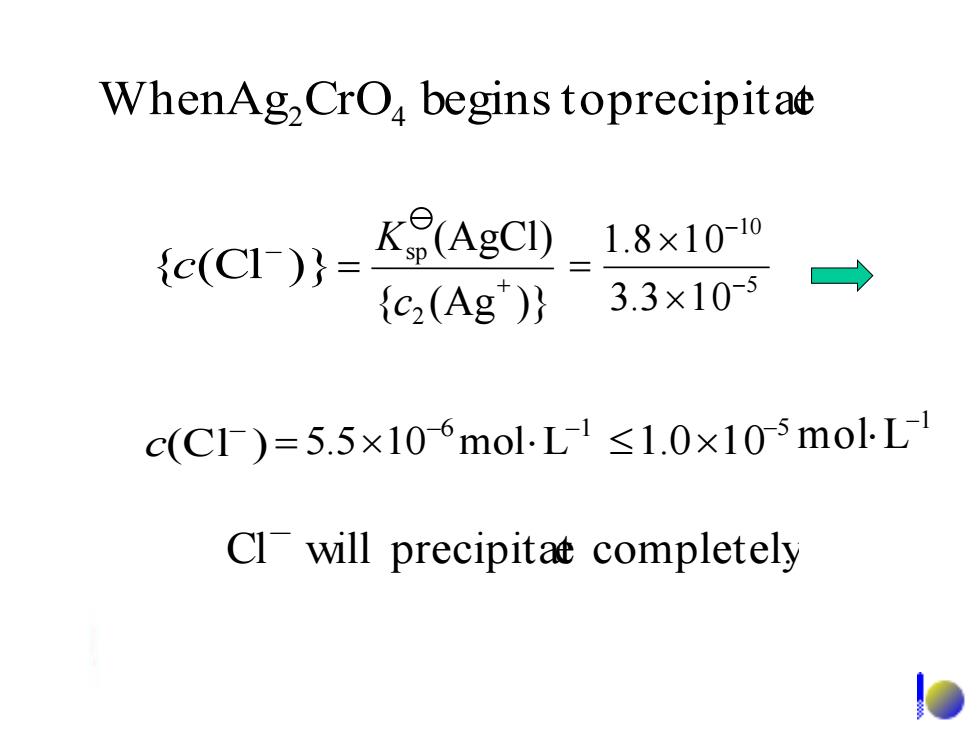
WhenAg,CrO begins toprecipitae {c(cI)}= K8(AgC_1.8x1010 {c2(Ag)} 3.3×10-5 c(C)=5.5×106mol.L1≤1.0×105molL1 Cl will precipitat completelyWhen Ag CrO begins toprecipitate 2 4 6 1 5.5 10 mol L - - = 5 1.0 10- 1 mol L - (Cl ) - c { (Cl )} - c 5 10 3.3 1 0 1.8 1 0 - - = { (Ag )} (AgCl) 2 sp + = c K Cl will precipitate completely. -