正在加载图片...
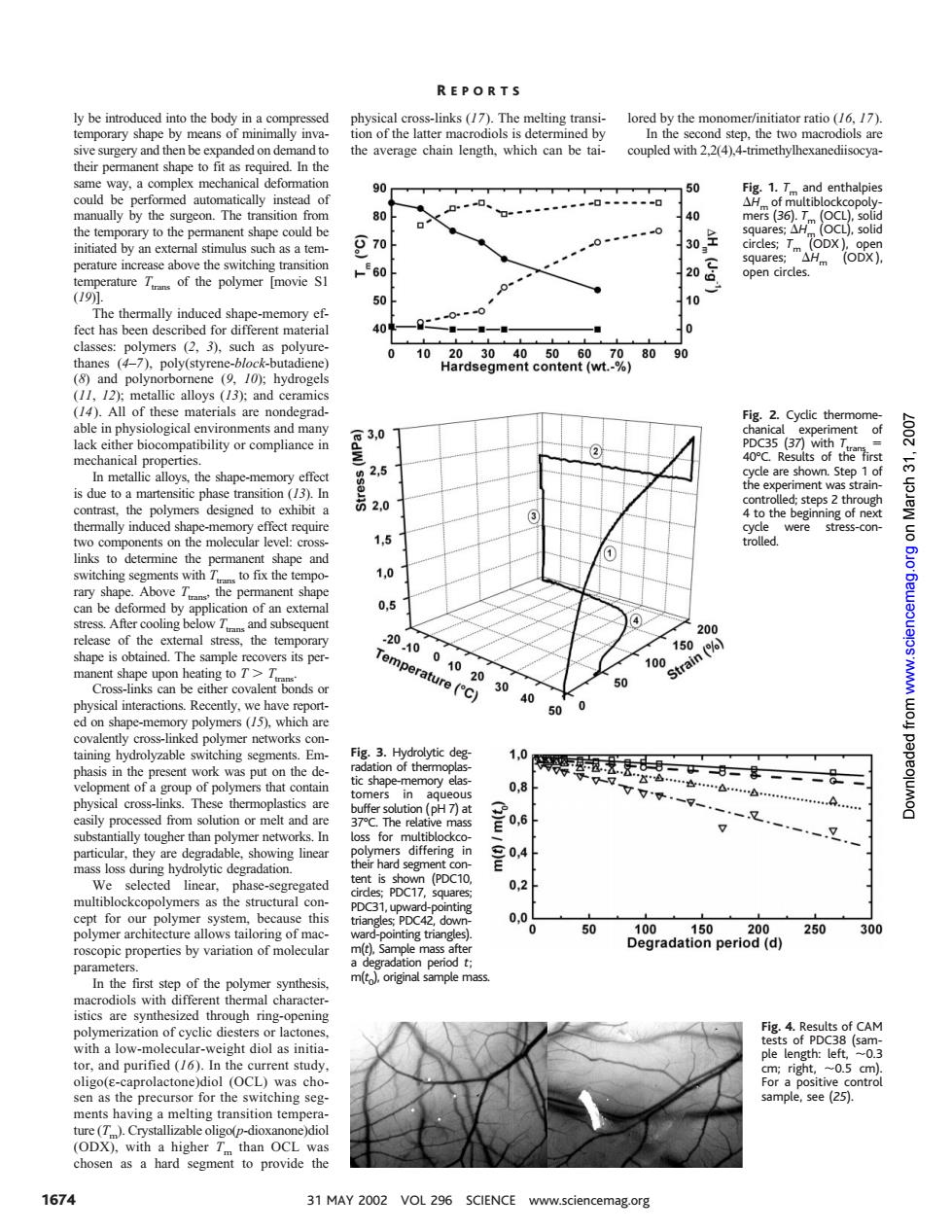
REPORTS nofhelarn mac 2.24 e o tep,the tw couple their pe nent shape to fit aseq ed.In the 50 Fig.1.and enthalpie gon 130全 by an external s as a tem 570 atureTof the polymer movie S 20 open circles. 10 he thermally i 101 90 All of t allic alloys(13):and c able inp logical environm ts and man 2 Cyclic therm lity or compliance i 3,0 (37)with 2.5 de are sh 0 is due toa m designed 4 to the b e of leve 1,5 the Ab 00 of th 20 -10 15 100 (% g 20 an 30 % (/5).which ar 40 esent work as pu 0.8 ()w 0.6 gher than in )w inea for our 50 Dradatlpenio9al 20 300 pic properties by variation of mole m In the first ster of the re cd山 on of CAM e the precurso for the sw ching se DX 1674 31 MAY 2002 VOL 296 SCIENCE www.sciencemag.orgly be introduced into the body in a compressed temporary shape by means of minimally invasive surgery and then be expanded on demand to their permanent shape to fit as required. In the same way, a complex mechanical deformation could be performed automatically instead of manually by the surgeon. The transition from the temporary to the permanent shape could be initiated by an external stimulus such as a temperature increase above the switching transition temperature Ttrans of the polymer [movie S1 (19)]. The thermally induced shape-memory effect has been described for different material classes: polymers (2, 3), such as polyurethanes (4–7), poly(styrene-block-butadiene) (8) and polynorbornene (9, 10); hydrogels (11, 12); metallic alloys (13); and ceramics (14). All of these materials are nondegradable in physiological environments and many lack either biocompatibility or compliance in mechanical properties. In metallic alloys, the shape-memory effect is due to a martensitic phase transition (13). In contrast, the polymers designed to exhibit a thermally induced shape-memory effect require two components on the molecular level: crosslinks to determine the permanent shape and switching segments with Ttrans to fix the temporary shape. Above Ttrans, the permanent shape can be deformed by application of an external stress. After cooling below Ttrans and subsequent release of the external stress, the temporary shape is obtained. The sample recovers its permanent shape upon heating to T . Ttrans. Cross-links can be either covalent bonds or physical interactions. Recently, we have reported on shape-memory polymers (15), which are covalently cross-linked polymer networks containing hydrolyzable switching segments. Emphasis in the present work was put on the development of a group of polymers that contain physical cross-links. These thermoplastics are easily processed from solution or melt and are substantially tougher than polymer networks. In particular, they are degradable, showing linear mass loss during hydrolytic degradation. We selected linear, phase-segregated multiblockcopolymers as the structural concept for our polymer system, because this polymer architecture allows tailoring of macroscopic properties by variation of molecular parameters. In the first step of the polymer synthesis, macrodiols with different thermal characteristics are synthesized through ring-opening polymerization of cyclic diesters or lactones, with a low-molecular-weight diol as initiator, and purified (16). In the current study, oligo(ε-caprolactone)diol (OCL) was chosen as the precursor for the switching segments having a melting transition temperature (Tm). Crystallizable oligo(p-dioxanone)diol (ODX), with a higher Tm than OCL was chosen as a hard segment to provide the physical cross-links (17). The melting transition of the latter macrodiols is determined by the average chain length, which can be tailored by the monomer/initiator ratio (16, 17). In the second step, the two macrodiols are coupled with 2,2(4),4-trimethylhexanediisocyaFig. 1. Tm and enthalpies DHm of multiblockcopolymers (36). Tm (OCL), solid squares; DHm (OCL), solid circles; Tm (ODX ), open squares; DHm (ODX ), open circles. Fig. 2. Cyclic thermomechanical experiment of PDC35 (37) with Ttrans 5 40°C. Results of the first cycle are shown. Step 1 of the experiment was straincontrolled; steps 2 through 4 to the beginning of next cycle were stress-controlled. Fig. 3. Hydrolytic degradation of thermoplastic shape-memory elastomers in aqueous buffer solution (pH 7) at 37°C. The relative mass loss for multiblockcopolymers differing in their hard segment content is shown (PDC10, circles; PDC17, squares; PDC31, upward-pointing triangles; PDC42, downward-pointing triangles). m(t), Sample mass after a degradation period t; m(t0), original sample mass. Fig. 4. Results of CAM tests of PDC38 (sample length: left, ;0.3 cm; right, ;0.5 cm). For a positive control sample, see (25). R EPORTS 1674 31 MAY 2002 VOL 296 SCIENCE www.sciencemag.org on March 31, 2007 www.sciencemag.org Downloaded from