正在加载图片...
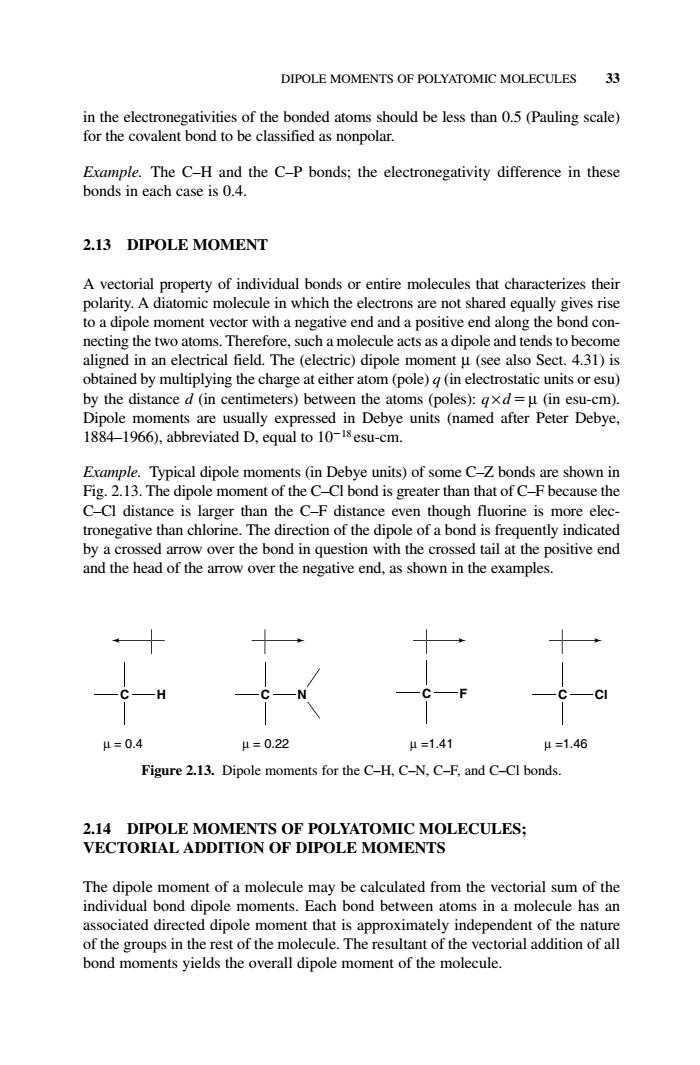
DIPOLE MOMENTS OF POLYATOMIC MOLECULES 33 sified as nonpolar. 2.13 DIPOLE MOMENT A vectorial property of individual bonds or entire molecules that characterizes their polarity.A diatomic molecule in which the electrons are not shared equally gives rise to a dipole moment vector with a negative end and a positive end along the bond con- necting the two atoms.Therefore.such a molecule acts as a dipole and tends to become aligned in an electrical field.The (electric)dipole moment u (see also Sect.4.31)is btained by multiplying the charge m (pole)(in electrostatic units or esu) by the distance(ce meters)betwe h atom (in esu-cm) are usu ly expres: are shown in C-CI distance is larger than the C-F distance even though fluorine is more elec- tronegative than chlorine.The direction of the dipole of a bond is frequently indicated by a crossed arrow over the bond in question with the crossed tail at the positive end and the head of the arrow over the negative end,as shown in the examples. =0d 4=0.22 1-141 Figure 2.13.Dipole moments for the C-H.C-N.C-F.and C-Cl bonds 2.14 DIPOLE MOMENTS OF POLYATOMIC MOLECULES; VECTORIAL ADDITION OF DIPOLE MOMENTS The dipole moment of a molecule may be calculated from the vectorial sum of the individual bo ond dipole n ents.Each bond betwee h ciateddirecteddipole of the moment tha appro the n t of the mo ue The resu t of the ve orial addition of alin the electronegativities of the bonded atoms should be less than 0.5 (Pauling scale) for the covalent bond to be classified as nonpolar. Example. The C–H and the C–P bonds; the electronegativity difference in these bonds in each case is 0.4. 2.13 DIPOLE MOMENT A vectorial property of individual bonds or entire molecules that characterizes their polarity. A diatomic molecule in which the electrons are not shared equally gives rise to a dipole moment vector with a negative end and a positive end along the bond connecting the two atoms. Therefore, such a molecule acts as a dipole and tends to become aligned in an electrical field. The (electric) dipole moment µ (see also Sect. 4.31) is obtained by multiplying the charge at either atom (pole) q (in electrostatic units or esu) by the distance d (in centimeters) between the atoms (poles): q × d µ (in esu-cm). Dipole moments are usually expressed in Debye units (named after Peter Debye, 1884–1966), abbreviated D, equal to 1018 esu-cm. Example. Typical dipole moments (in Debye units) of some C–Z bonds are shown in Fig. 2.13. The dipole moment of the C–Cl bond is greater than that of C–F because the C–Cl distance is larger than the C–F distance even though fluorine is more electronegative than chlorine. The direction of the dipole of a bond is frequently indicated by a crossed arrow over the bond in question with the crossed tail at the positive end and the head of the arrow over the negative end, as shown in the examples. 2.14 DIPOLE MOMENTS OF POLYATOMIC MOLECULES; VECTORIAL ADDITION OF DIPOLE MOMENTS The dipole moment of a molecule may be calculated from the vectorial sum of the individual bond dipole moments. Each bond between atoms in a molecule has an associated directed dipole moment that is approximately independent of the nature of the groups in the rest of the molecule. The resultant of the vectorial addition of all bond moments yields the overall dipole moment of the molecule. DIPOLE MOMENTS OF POLYATOMIC MOLECULES 33 C H C N C F C Cl µ = 0.4 µ = 0.22 µ =1.41 µ =1.46 Figure 2.13. Dipole moments for the C–H, C–N, C–F, and C–Cl bonds. c02.qxd 5/17/2005 5:13 PM Page 33��