正在加载图片...
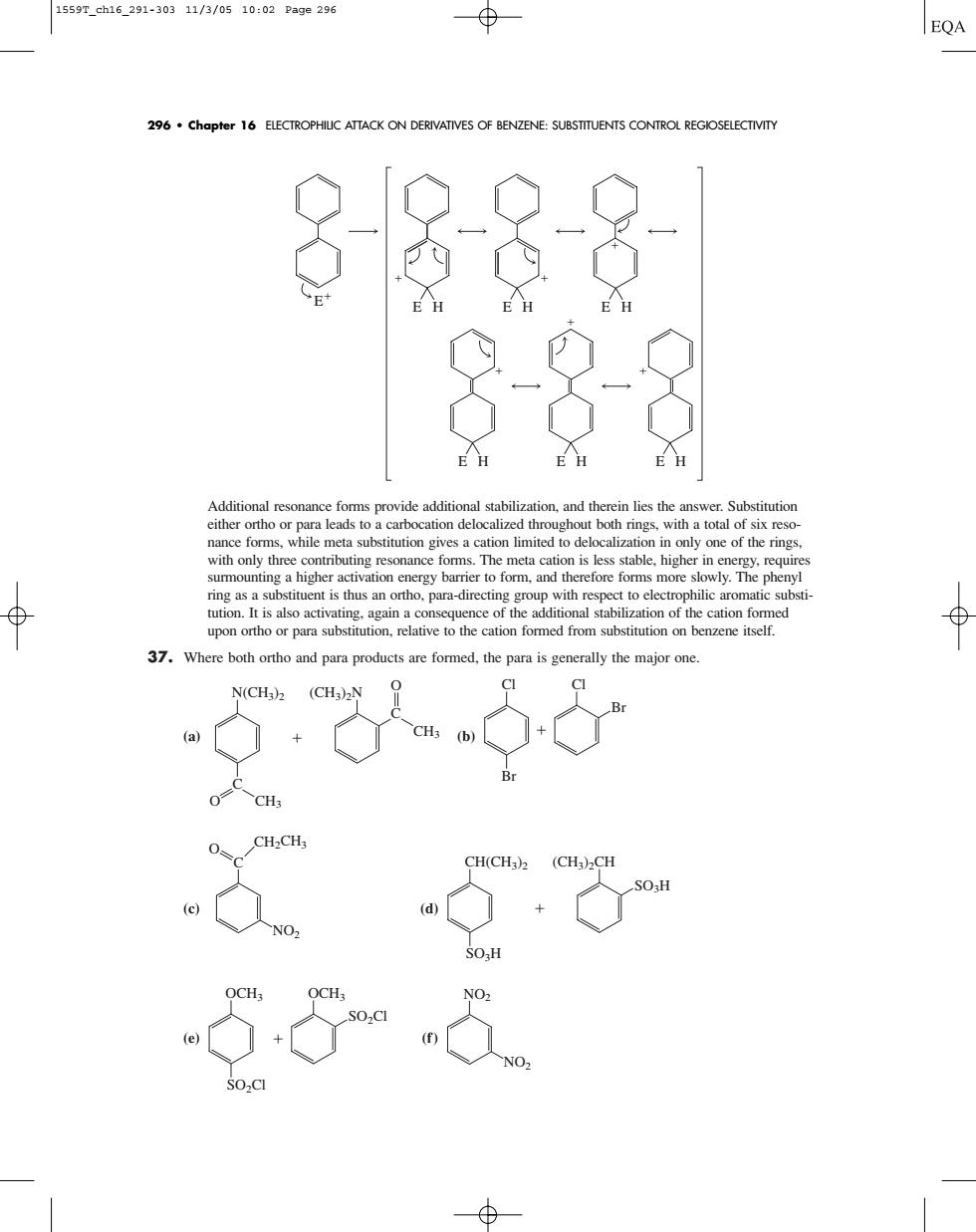
1559T_ch16_291-30311/3/0510:02Page296 ⊕ EQA 296.chapter 16 ELECTROPHILIC ATTACK ON DERIVATIVES OF BENZENE:SUBSTITUENTS CONTROL REGIOSELECTIVITY 6888 8月 Additional reso e forms provide additional stabilization.and therein lies the answer.Substitution one of the rig nng as a tuent is thus an ortho.para-directing group with respect to el Where both ortho and para products are formed,the para is generally the Additional resonance forms provide additional stabilization, and therein lies the answer. Substitution either ortho or para leads to a carbocation delocalized throughout both rings, with a total of six resonance forms, while meta substitution gives a cation limited to delocalization in only one of the rings, with only three contributing resonance forms. The meta cation is less stable, higher in energy, requires surmounting a higher activation energy barrier to form, and therefore forms more slowly. The phenyl ring as a substituent is thus an ortho, para-directing group with respect to electrophilic aromatic substitution. It is also activating, again a consequence of the additional stabilization of the cation formed upon ortho or para substitution, relative to the cation formed from substitution on benzene itself. 37. Where both ortho and para products are formed, the para is generally the major one. (a) (b) (c) (d) (e) (f) NO2 NO2 OCH3 OCH3 SO2Cl SO2Cl CH(CH3)2 (CH3)2CH SO3H SO3H C NO2 O CH2CH3 Cl Cl Br Br C N(CH3)2 (CH3)2N O CH3 C CH3 O E E H E H E H E H E H E H 296 • Chapter 16 ELECTROPHILIC ATTACK ON DERIVATIVES OF BENZENE: SUBSTITUENTS CONTROL REGIOSELECTIVITY 1559T_ch16_291-303 11/3/05 10:02 Page 296�����������