正在加载图片...
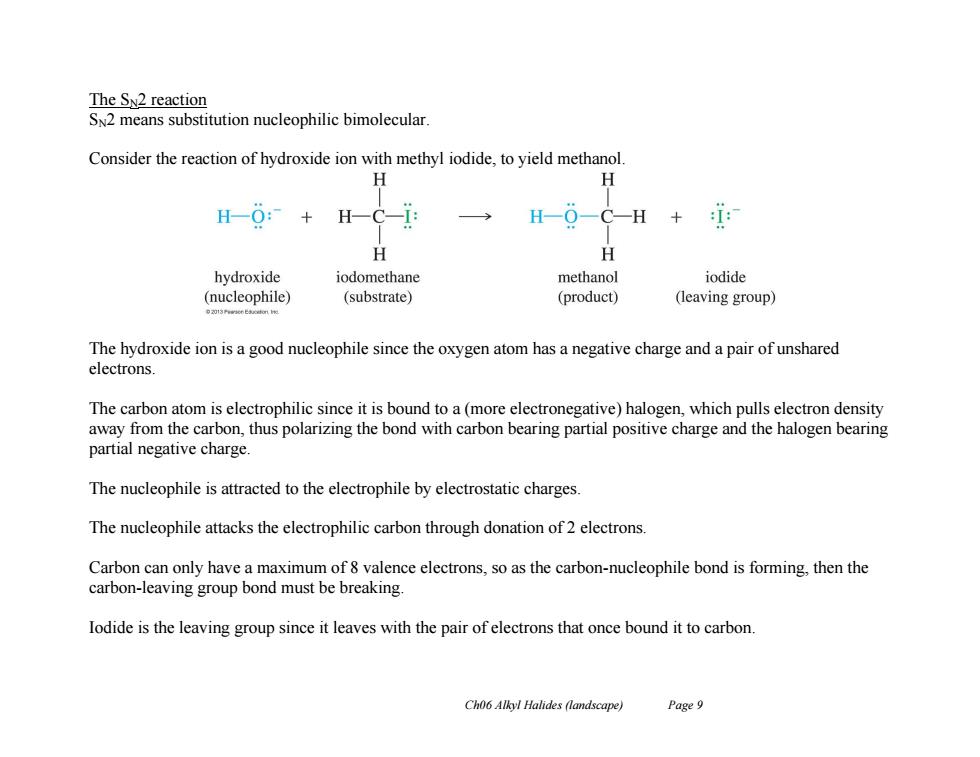
The SN2 reaction SN2 means substitution nucleophilic bimolecular. Consider the reaction of hydroxide ion with methyl iodide,to yield methanol. H H H0:+ H H一O一C—H+ H H hydroxide iodomethane methanol iodide (nucleophile) (substrate) (product) (leaving group) The hydroxide ion is a good nucleophile since the oxygen atom has a negative charge and a pair of unshared electrons The carbon atom is electrophilic since it is bound to a(more electronegative)halogen,which pulls electron density away from the carbon,thus polarizing the bond with carbon bearing partial positive charge and the halogen bearing partial negative charge. The nucleophile is attracted to the electrophile by electrostatic charges The nucleophile attacks the electrophilic carbon through donation of 2 electrons. Carbon can only have a maximum of 8 valence electrons,so as the carbon-nucleophile bond is forming,then the carbon-leaving group bond must be breaking. lodide is the leaving group since it leaves with the pair of electrons that once bound it to carbon. Ch06 Alkyl Halides (landscape) Page 9Ch06 Alkyl Halides (landscape) Page 9 The SN2 reaction SN2 means substitution nucleophilic bimolecular. Consider the reaction of hydroxide ion with methyl iodide, to yield methanol. The hydroxide ion is a good nucleophile since the oxygen atom has a negative charge and a pair of unshared electrons. The carbon atom is electrophilic since it is bound to a (more electronegative) halogen, which pulls electron density away from the carbon, thus polarizing the bond with carbon bearing partial positive charge and the halogen bearing partial negative charge. The nucleophile is attracted to the electrophile by electrostatic charges. The nucleophile attacks the electrophilic carbon through donation of 2 electrons. Carbon can only have a maximum of 8 valence electrons, so as the carbon-nucleophile bond is forming, then the carbon-leaving group bond must be breaking. Iodide is the leaving group since it leaves with the pair of electrons that once bound it to carbon