正在加载图片...
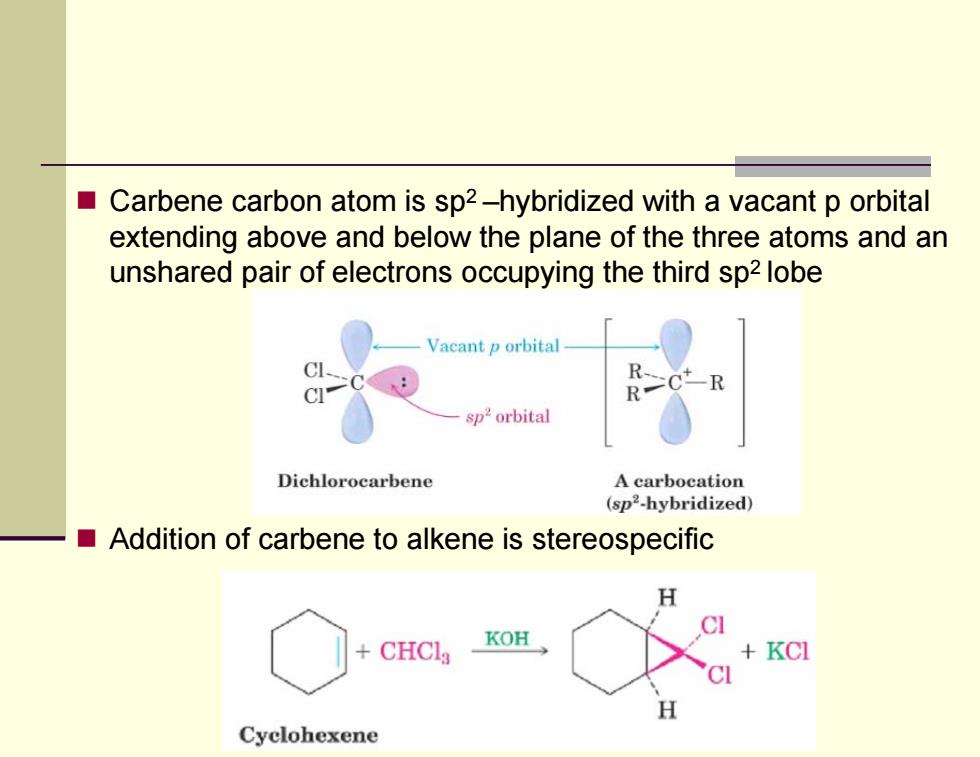
■ Carbene carbon atom is sp2-hybridized with a vacant p orbital extending above and below the plane of the three atoms and an unshared pair of electrons occupying the third sp2 lobe Vacant p orbital R、 -R sp2orbital Dichlorocarbene A carbocation (sp2-hybridized) Addition of carbene to alkene is stereospecific CHCla KOH KCI H Cyclohexene Carbene carbon atom is sp2 –hybridized with a vacant p orbital extending above and below the plane of the three atoms and an unshared pair of electrons occupying the third sp2 lobe Addition of carbene to alkene is stereospecific